FINAL DIAGNOSIS
Rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory, autoimmune disease of unknown etiology which primarily affects synovial joints.1 The flagship feature of RA is persistent symmetric polyarthritis beginning in the joints of the hands and feet. Extra-articular manifestations of RA in the skin, heart, lungs, and eyes can be significant. In general, pathology of the small joints in the hands and feet are the first to be affected by RA. The most common joints involved are the metacarpophalangeal (MCP), wrist, proximal interphalangeal (PIP), knee, metatarsophalangeal (MTP), but may also include the shoulder, ankle, cervical spine, hip, elbow, and temporomandibular joints.2,3 The acutely affected joints show inflammation with swelling, tenderness, and warmth, and pain with decreased range of motion. Additional findings such as atrophy of the interosseous muscles of the hands are seen towards the beginning of the disease process. Deformities such as ulnar deviation, boutonniere, swan-neck, and hammertoe deformities are later manifestations of the disease secondary to repeated joint injury and tendon destruction. The disease usually progresses from the peripheral to more proximal joints and results in significant disability within 10 to 20 years in patients without adequate disease control. Spontaneous remission from RA is uncommon, especially after the first 3-6 months.
Rheumatoid Arthritis Diagnostic Criteria
The 2010 American College of Rheumatology/European League Against Rheumatism developed an updated classification system for the diagnosis of RA.4 Historically, RA was diagnosed based on the persistence of arthritic symptoms over time; however, this classification system failed to identify patients early in the disease course. It is now recognized that very early aggressive therapeutic intervention with disease-modifying antirheumatic drugs (DMARDs) significantly improves clinical outcomes, reduces irreversible joint damage and disability, and provides the best opportunity to achieve disease remission (the ideal window for intervention is defined as symptom duration < 12 weeks prior to the initiation of treatment). According to the new criteria, any patient who has at least one joint with definitive clinical synovitis in which the synovitis is not better explained by another disease process (such as gout, lupus or psoriatic arthritis) should be assessed for RA.4 The new classification system defines four categories for diagnosis and awards points based on defined criteria. The maximum number of points possible is 10. A classification of definitive RA requires a score of at least 6/10.4 Patients who do not reach the threshold of diagnosis, but still present a clinical concern can be reassessed over time.
The new classification criteria include:
Joint involvement (0-5 points):
At least 1 serology test result is needed for RA classification (0-3 points):
At least 1 test acute-phase reactant test result is needed for classification (0-1 point):
Points allocated based on the patient's self-reporting of the duration of signs or symptoms of synovitis in clinically involved joints (0-1 point):
The incidence of RA is two to three-fold higher in women than men. The average age of onset in both sexes occurs in the 6th decade of life. The lifetime risk of rheumatoid arthritis has been estimated to be approximately 4% among women and 3% among men. First-degree relatives of patients with RA have a two to three-fold higher risk of developing RA. There is significant evidence that specific HLA class II genotypes are associated with an increased risk for developing RA, with the strongest association found with DRB1*0401 and DRB1*0404 alleles.5 Many other environmental factors likely contribute to the pathogenesis of RA and the full picture of the risk factors associated RA has yet to be elucidated.
Laboratory Testing
Laboratory testing for RA can be complex and extensive. It may include antinuclear antibody (ANA) with reflex testing (negative ANA helps exclude other systemic rheumatic diseases; though up to one-third of patients with RA may have a positive ANA), complete blood count (CBC) with differential and platelet count (CBC often shows anemia and thrombocytosis in RA), serum uric acid, urinalysis, and synovial fluid analysis (for the diagnosis or exclusion of gout, pseudogout, or an infectious arthritis), serology including rheumatoid factor and anti-CCP antibody (RF is seen in up to 80% of patients with RA, anti-CCP antibodies have a similar sensitivity for RA but may be detected earlier and generally have a much higher specificity, up to 95-98% with high titer), and acute phase reactants such as ESR and CRP (with the caveat that these tests are serving as non-specific markers for inflammation).6,7,8,9
Clinical Management
The current guidelines support early aggressive treatment with DMARDs. Methotrexate is the first-line therapy for the treatment of RA and prednisone is often added at the onset of acute disease to ameliorate severe inflammation until the methotrexate is therapeutic. Combining methotrexate with a biologic medications, such as the monoclonal antibody adalimumab (anti-TNF agent), have been shown to work synergistically and provide better symptom relief with decreased disease progression than using either agent alone.10,11,12
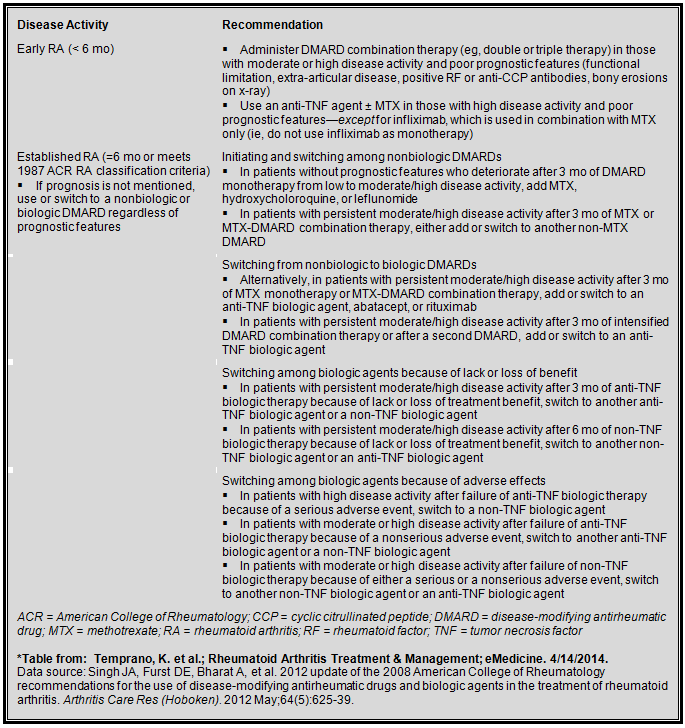
Medication regimens for early and established rheumatoid arthritis13

Case Resolution
The patient's PPD was negative and the decision was made to add adalimumab to her methotrexate regimen. The patient's symptoms resolved and repeated CRP and sedimentation rates decreased to within reference range.
References
![]() Contributed by Michelle Stram, MD, ScM and Bruce Rabin, MD, PhD
Contributed by Michelle Stram, MD, ScM and Bruce Rabin, MD, PhD