FINAL DIAGNOSIS
Mast cell activation syndrome (secondary)
Introduction
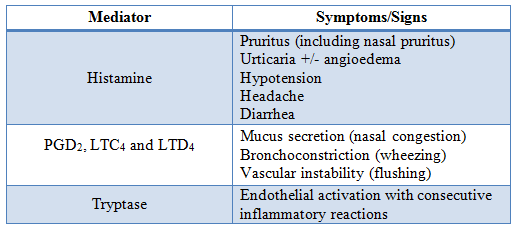
Mast cell activation syndromes are a broad group of disorders with clinical symptoms ranging from mild pruritus and nausea to anaphylaxis.1 These syndromes are due to systemic release of mast cell mediators including histamine, prostaglandin D2, prostaglandin F2, cysteinyl leukotrienes (LTC4, D4, and E4), and tryptase as well as many cytokines and chemokines.1,2 A summary of several mediators and the symptoms which they cause are included in Table 2.
Table 2. Common mast cell mediators and the symptoms they produce. Modified from Valent et al, 2012.2

The symptoms described in mast cell activation syndromes are nonspecific and can be present in a variety of other disorders.1 A consensus panel was recently convened in order to better classify the underlying causes of mast cell activation and to clearly define diagnostic criteria for mast cell activation.2
Mast cell activation diagnostic criteria
Each of the criteria is meant to be taken in context of the others, as no one criterion is enough to clearly identify mast cell activation.2
Syndrome Classification
Primary mast cell activation disorder includes Mastocytosis and monoclonal mast cell activation syndrome. Mastocytosis is a rare but well-recognized and well-defined entity which is subdivided into localized mast cell tumors, cutaneous Mastocytosis, and systemic Mastocytosis. The diagnosis of systemic Mastocytosis according to the WHO requires the presence of one major and one minor or three minor criteria. One of the minor criteria is the presence of a common point mutation in c-KIT at codon 816.3 Monoclonal mast cell activation syndrome is diagnosed in patients who have a mast cell population with a c-KIT mutation but do not meet the necessary diagnostic criteria for Mastocytosis. Patients with Mastocytosis and monoclonal mast cell activation syndrome do not necessarily develop symptoms of mast cell activation.2,3
Secondary mast cell activation syndrome includes patients who experience mast cell activation with identifiable triggers. These include IgE-mediated allergies, some variants of physical and chronic urticaria, and select neoplastic and hematologic disorders. IgE-mediated allergens include food, drugs, and hymenoptera stings among many others. Exercise-induced anaphylaxis and food associated exercise-induced anaphylaxis are included amongst the physical urticaria associated with these syndromes.1,2
Idiopathic mast cell activation syndromes are those for which no identifiable cause can be identified. The categories within this group include idiopathic mast cell activation syndrome, idiopathic urticaria, idiopathic angioedema, and idiopathic anaphylaxis. These are diagnoses of exclusion where the criteria for mast cell activation have been met, but no primary or secondary cause is identified.2
Laboratory Testing
Serum tryptase is currently the most widely used marker for mast cell activation. It accounts for 20% of the mast cell protein content. It is also produced by basophils at a much lower volume.4 Mast cell release of tryptase is proportional to the release of histamine yet has a longer half-life (2 hours) than histamine (2 minutes).4,5 Persistent baseline tryptase levels greater than 20 ng/mL is considered a minor criterion for the diagnosis of systemic Mastocytosis except in the presence of non-mast cell lineage clonal disease.3 Baseline serum tryptase levels have also been shown to be elevated in a subset of patients with idiopathic mast cell activation.6 Patients with an elevated serum tryptase are also more likely to develop more severe anaphylactic reactions compared to those with normal serum tryptase.7 The consensus guidelines for mast cell activation indicate that the baseline tryptase level should increase by 20% plus 2 ng/mL during or directly after an acute event. For example, a patient with a baseline tryptase of 8 would have a tryptase level increase to at least 11.6 ng/mL (8 + 8*0.2 +2).2
Many of the most recognizable symptoms of mast cell activation are caused by histamine and prostaglandin D2. The use of histamine as a marker of mast cell activation, however, is complicated by the fact that basophils also secrete significant amounts of histamine.4 Serum testing of both factors is limited by their short half-life. An alternative is measuring 24-hour urine levels of these compounds or their metabolites. The levels of these metabolites have been shown to rise in response to mast cell activation.4,8 A recent study found that even baseline levels of 24-hour urine 11β-Prostaglandin2α are elevated in patients with idiopathic mast cell activation syndrome, while levels of 24-hour urine N-methyl histamine were not significantly increased.6 There are no current consensus guidelines regarding the level of increase of serum or urine markers of mast cell activation other than tryptase.2
Clinical Management
Treatment of mast cell activation consists of stepwise increases in medication starting with H1-antihistamine for general symptoms and a H2-antihistamine for prominent gastrointestinal symptoms. The second phase includes the addition of leukotrienes for general symptoms and oral cromolyn sodium for gastrointestinal symptoms. Beyond this level of treatment strong evidence for mast cell activation is recommended. Further treatment includes glucocorticoids, ketotifen, hydroxychloroquine, dapsone, cyclosporine, and omalizumab.1,2 For patients with Mastocytosis meeting WHO diagnostic criteria cytoreductive therapy with alpha interferon or cladribine is sometimes initiated. Patients with suspected mast cell activation are also given injectable epinephrine for use in case of emergency.1 Patients with elevated prostaglandin have had symptom improvement with aspirin therapy.6
Case Resolution
Given the association of the patient's symptoms with exercise, pressure, and hot showers it is likely that she is experiencing a physical urticaria, a type of secondary mast cell activation syndrome. She meets two of the mast cell activation syndrome diagnostic criteria: symptoms occurring in two or more organ systems (cutaneous and GI) and response of the symptoms to anti-histamines.2 The presence of a transient increase in mediators has not yet been determined as the patient's laboratory samples were obtained while she was maintained on anti-histamine medications. Additionally, there is no indication that they were obtained during or directly after an acute episode. The patient's baseline tryptase <20 ng/ML is reassuring that the patient does not have an underlying primary mast cell activation syndrome.3 Given the response of symptoms to anti-histamines, the patient was advised to continue taking ranitidine prior to exercise, though she was switched from fexofenadine to cetirizine given her pregnancy. Due to the risk of anaphylaxis, she was prescribed injectable epinephrine and was advised not to exercise alone.
REFERENCES
![]() Contributed by Michelle Heayn, MD, PhD and Octavia M. Peck Palmer, PhD
Contributed by Michelle Heayn, MD, PhD and Octavia M. Peck Palmer, PhD