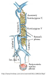
FINAL DIAGNOSIS Mycobacterium bovis
DISCUSSION
Mycobacterium bovis is a member of the mycobacterium tuberculosis complex, that also includes M. tuberculosis, M. africanum, M. microti, M. canetti. M. bovis is typically the cause of tuberculosis in cattle, deer, and other such mammals. However, a bacillus capable of infecting humans has developed, possibly due to animal domestication, and causes 1-2% of human tuberculosis cases in developed countries. Transmission typically occurs by inhaling aerosolized respiratory droplets from infected cattle or by direct contact with other animals such as elk, deer, and seals. However, infection may also be due to consumption of infected cow's milk products, Bacille Calmette-Guérin (BCG) vaccination for TB prevention, and intravesical BCG installation for bladder cancer therapy. M. bovis tuberculosis is clinically and radiographically indistinguishable from tuberculosis due to M. tuberculosis, but has a worse prognosis. It is treated with the standard anti-tuberculosis drugs except for pyrazidamine, due to inherent resistant.1
BCG is a live-attenuated strain of M. bovis. It was first used for immunization against tuberculosis in 1921 and in 1976, it was introduced as an intravesical treatment for superficial bladder cancer. It has continued to be used for the treatment of and prophylaxis against Ta and T1 tumors and carcinoma in situ. The exact antitumor mechanism is not known, but it is believed to trigger a variety of local immune responses including a mononuclear cell infiltrate that is composed mainly of CD4 T cells and macrophages. This inflammatory infiltrate is associated with appearance of interferon-gamma causing an increase in urine cytokine levels following treatment. This therapy has been shown to delay (although not necessarily prevent) tumor progression to a more advanced stage, decrease the need for subsequent cystectomy, and improve overall survival.2,3
As with any therapy, intravesical BCG therapy has complications, however the exact pathogenesis of these complications is not understood. It is proposed that it may be due to a hypersensitivity reaction because of the presence of granulomas, absence of recoverable organisms, and clinical response to glucocorticoids when given with anti-tuberculosis drugs. It has also been proposed that the complications arise as a result of an ongoing active infection as few case reports demonstrate isolation of viable organisms from a variety of tissues. It believed that the organism gains access to lymphatics and blood via disruption of uroepithelial cells and disseminate to a variety of sites.2,3
The most common complication is BCG cystitis which is marked by dysuria and increased frequency. There is the potential for the infection to extend into the ureter, urethra, prostate, or epididymis. Up to 20% of patients receiving intravesical BCG also develop a conventional bacterial UTI at some point which must be treated appropriately.2,3
More severe systemic complications have been reported that include sepsis, granulomatous hepatitis, and osteomyelitis.4-5 The majority of the osteomyelitis cases involve the spine.6-10 It is proposed that BCG spreads from the urinary tract to the spine via Batson's venous plexus which, in addition to the spread of infection, also serves as a route for metastatic spread for malignancies (Figure 4). Other rare complications that have been reported include mycotic aneurysms, psoas muscle abscess, and endopthalmitis. 11-13
The current recommendations for identifying mycobacterial organisms include a combination of phenotypic and molecular assays.1 In this case, the organism was initially detected by culture using liquid broth media. Later, the organism grew small, buff colonies on solid Lowenstein-Jensen media. An acid-fast stain was applied to the organisms that grew in culture in order to visualize and confirm the growth of acid-fast organisms. Two DNA probes, one for the M. tuberculosis complex and another for the M. avium/M. intracellulare complex, were used to further categorize the organism. The organism was identified to be a member of M. tuberculosis complex. To further speciate the organism, drug susceptibility testing was performed and demonstrated pyrazinamide resistance. Biochemical tests showed that the organism was negative for niacin accumulation and did not reduce nitrate to nitrite. The organism was then sent to the Department of Health where genotyping confirmed the identity. The combined results of these various assays allowed for the proper identification of M. bovis in this case of vertebral osteomyelitis.
REFERENCES
![]() Contributed by Stacey Barron, MD and William Pasculle, ScD
Contributed by Stacey Barron, MD and William Pasculle, ScD