FINAL DIAGNOSIS
HUMAN ANTI-MOUSE ANTIBODIES (HAMAs) INTERFERE WITH THYROGLOBULIN MEASUREMENTS IN SERUM
DISCUSSION
Thyroglobulin
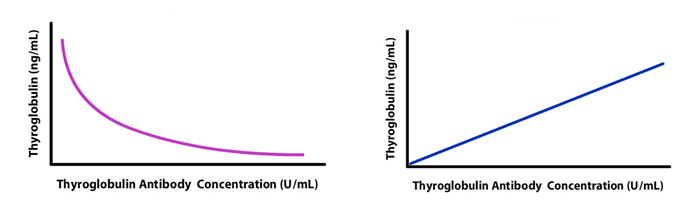
The thyroid is an endocrine gland that is the only source of thyroglobulin (MW 660,000 kDa) in the body (1). Thyroglobulin is made by both normal thyroid follicular cells and differentiated thyroid cancer cells (2). Serum thyroglobulin is used as a tumor marker to monitor patients with differentiated thyroid cancer pre and postoperatively. It is common clinical practice to measure both thyroglobulin and thyroglobulin antibody because antibodies to thyroglobulin may negatively or positively interfere with the accurate measurement of thyroglobulin in serum (Figure 2). This is of great concern because thyroglobulin antibodies have been reported to be present in 10 % of the general population and in 20% of patients with differentiated thyroid cancer (3). A falsely increased thyroglobulin result may prompt the clinician to recommend the patient to undergo unnecessary therapeutic interventions (e.g. radiation ablation, surgery) whereas a falsely decreased thyroglobulin result may mask a diagnosis of disease recurrence.
Antibody interference can be suspected in several settings

Figure 2: The Effects of Endogenous Thyroglobulin Antibody on the Measurement of Serum Thyroglobulin using Immunoenzymatic (Sandwich) Method
Thyroglobulin Immunoassays
Several immunoassays are commercially available to measure thyroglobulin including the competitive radioimmunoassay (RIA) and the one-step immunoenzymatic (sandwich) assay (IMA). The RIA method is older and more labor intensive compared to IMA. In the RIA method, thyroglobulin competes with 125I labeled human thyroglobulin for a rabbit antibody against human thyroglobulin. After six days of incubation, thyroglobulin - antibody complexes are precipitated with a second anti-rabbit antibody. This method can detect both free thyroglobulin and thyroglobulin bound to antibody. The radioactivity is inversely proportional to the thyroglobulin concentration (2). In contrast to RIA, the IMA can be performed within one hour. The thyroglobulin in the patient's sample is mixed with a biotinylated reagent that contains four monoclonal anti-thyroglobulin antibodies, streptavidin coated paramagnetic particles and monoclonal anti- thyroglobulin antibody alkaline phosphatase conjugate. Patient thyroglobulin and the biotinylated antibodies bind the solid phase and the conjugate antibody reacts with a different antigenic site on the thyroglobulin molecule. Following a wash, chemiluminescent substrate is added and the generated light is directly proportional to the thyroglobulin concentration in the serum (1). The IMA method is generally more prone to thyroglobulin antibody interference compared to the RIA method (2). However, no method is completely exempt from interference when measuring thyroglobulin in the presence of antibodies (3).
Case Review
An athyreotic patient should have no detectable serum thyroglobulin. Since this patient underwent total thyroidectomy, these thyroglobulin results were inconsistent with the clinical scenario and prompted the reference laboratory to suspect the presence of an interferant in the serum. Both serum samples were treated with blocking reagent that contained mouse immunoglobulins (Scantibodies Laboratory, Inc). Following treatment with the blocking reagent, the thyroglobulin concentrations were undetectable in both samples (<0.1 ng/mL). This confirmed that HAMAs were present in the sample. In this case HAMAs and not thyroglobulin antibodies were responsible for the falsely elevated thyroglobulin concentrations in this athyreotic patient (4). Sufficient sample was not available to perform confirmatory thyroglobulin testing via the RIA method to determine if the thyroglobulin antibodies would interfere using this methodology. The identification of antibodies (HAMAs) are important because they can interfere with the accurate measurements of a variety of serum analytes (e.g. troponin, ß-hCG, tumor markers) when using immunoenzymatic methods (1).
HAMAs
HAMAs are defined as human antibodies to mouse antibodies and have been demonstrated to interfere with 3% of thyroglobulin testing (2). Human antibodies that react with animal proteins have been implicated as an unsuspected source of interference in immunoassays. The theory is that these anti-animal antibodies arise from exposure to animals (direct contact with animals or ingesting animal derived pharmaceuticals) (4). We are not aware of how our patient developed HAMAs. Commonly, false positive results occur when HAMAs interfere with sandwich immunoassays by binding to the animal derived capture antibody. Less frequently HAMAs account for false negative results. This occurs due to the lack of antibody-antigen complexes because the HAMA saturates the capture antibody and conjugate (1).
REFERENCES
![]() Contributed by Lisa Radkay, MD and Octavia Peck Palmer, PhD
Contributed by Lisa Radkay, MD and Octavia Peck Palmer, PhD