Final Diagnosis -- Wild-type MTHFR 677 genotype
FINAL CONCLUSION
No added benefit in performing additional MTHFR gene mutation analysis. However, other genetic tests can be recommend, see discussion.
DISCUSSION
MTHFR (5,10-methylenetetrahydrofolate reductase) is an enzyme which catalyzes the conversion of 5,10-methylene-tetrahydrofolate (5,10-methylene-THF) to 5-methyl-tetrahydrofolate (5-methyl-THF). [Image 1] 5-methyl-tetrahydrofolate can methylate homocysteine to form methionine. Methionine can then be activated to AdoMet (S-adenosylmethionine), which serves as a methyl donor to multiple cellular components, including nucleic acids, lipids, proteins, and neurotransmitters (1). 5,10-methylenetetrahydrofolate, on the other hand, can be converted to 10-formyl-tetrahydrofolate (10-formyl-THF), which is involved in the metabolism of folate and the synthesis of purines and pyrimidines.
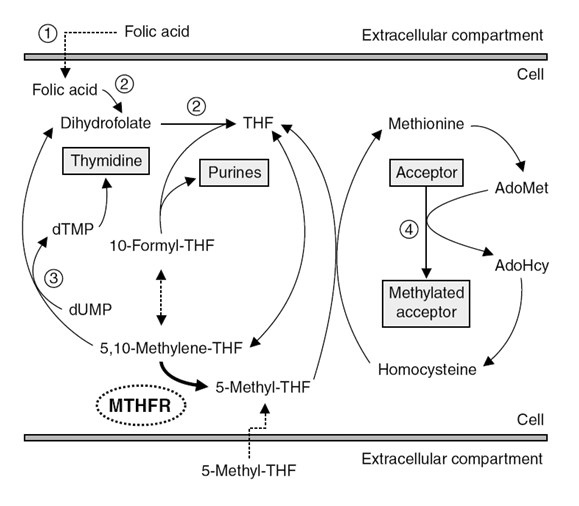
[Figure 1.. Schwahn B, Rozen R. Polymorphisms in the methylenetetrahydrofolate reductase gene: clinical consequences. Am J Pharmacogenomics. 2001;1(3):189-201.]
What are described nucleotide sequence variants in the MTHFR gene?
Severe MTHFR deficiency results most frequently from missense mutations; however, nonsense, splice site and deletion mutations have also been described (2). Twenty-four different mutations have been described, and these occur at different locations from those in mild MTHFR deficiency. Severe deficiency is defined as <20% residual enzymatic activity in cultured fibroblasts or as a plasma homocysteine level more than 10-fold greater than normal.
In mild MTHFR deficiency, the most commonly-associated variant is the C->T sequence change at nucleotide 677, called MTHFR C677T (standard nomenclature c.665C>T). This change results in a missense change, an alanine to valine substitution, at codon 222. As a consequence, the MTHFR enzyme is thermolabile and may be associated with mildly increased plasma homocysteine levels (1). This variant is commonly found in North American, European, and Asian populations, with the highest frequencies among Southern Mediterranean and Hispanic populations in North America (2). Sub-Saharan African and African Americans have much lower frequencies (3). The C677T variant has a homozygosity frequency of up to 30%. Patients with homozygous MTHFR C677T can have moderate (16-30umol/L) or intermediate (31-100umol/L) hyperhomocysteinemia. A second common MTHFR variant is the A>C transition at nucleotide position 1298, also known as A1298C (standard nomenclature c.1286). This change results in a missense glutamine to alanine substitution at codon 429. The resulting MTHFR molecule is not thermolabile and does not affect enzyme methylation in in vitro studies like MTHFR C677T (4).
What are the disease associations with MTHFR mutations?
Severe MTHFR deficiency
- Patients with severe MTHFR deficiency typically have severe hyperhomocystinuria (greater than 100umol/L), hypomethioninemia, decreased circulating folate, decreased CH3-tetrahydrofolate, and decreased neurotransmitters (1). As a consequence, they may have neurologic and psychiatric symptoms due to neuronal loss and encephalopathy. In addition, they may have vasculopathy and coagulopathy, including thromboembolic disease. Histologically, they will show vascular changes such as intimal hyperplasia, variable vessel fibrosis and disruption of elastic laminae in arterial walls (2). These changes reflect response to damage caused by high levels of homocysteine.
Mild MTHFR deficiency
- In mild MTHFR deficiency, patients may have premature coronary artery disease and cerebral infarction (3). The mechanism by which vessel disease occurs is thought to be related to elevated homocysteine levels. Jacovina and colleagues reported a unique in vivo mechanism suggesting that an annexin A2-deficient mouse rendered hyperhomocysteinemic by dietary means demonstrated impaired fibrinolysis, perivascular fibrin persistence, and attenuated angiogenesis (angiostasis) (4). This group had also previously reported that homocysteine impairs endothelial cell surface plasminogen activation by posttranslationally modifying annexin A2, the coreceptor for plasminogen and tissue plasminogen activator.
- Some studies suggest that elevated homocysteine levels may play a role in venous thrombosis. Lievers assessed homocysteine levels in patients with MTHFR 677 and 1298 variants and found a significant increase in homocysteine levels with both MTHFR 677CT and 677TT, but no change in fasting or methionine loaded homocysteine levels with MTHFR 1298 mutations (5). Further, homozygous MTHFR 677TT appeared to be synergistic with heterozygous mutations of coagulation factors Factor V or prothrombin for the development of deep venous thrombosis or pulmonary embolisms. However, the rate of wild-type MTHFR 677 in patients with venous thromboembolism was the same as that compared to the control healthy patients (6). These studies suggest that MTHFR 677 polymorphism can result in elevated homocysteine levels, which may lead to an increased risk of thrombotic events.
- However, there is evidence which more strongly supports that common MTHFR variants are not a prothrombotic genetic factor. Akar showed that patients with MTHFR 677 and 1298 polymorphisms in homozygous, single heterozygous, or double heterozygous combinations do not have an increased incidence of deep venous thromboses. His study did find that when one MTHFR variant, either 677 or 1298, is present (homozygous or heterozygous s) with Factor V Leiden, then there is significant association with venous thrombosis. A study by Franco found no role for MTHFR 1298 in the predisposition to DVT, either alone or with coinheritance of the Factor V Leiden, Factor II mutations or the MTHFR 677 variant. Spiroski also found no association of MTHFR 677 or 1298 variants with occlusive arterial disease or deep venous thrombosis. And Meisel, who performed a large scale study analyzing effects of the MTHFR 677/1298 variants, showed no significant increase in homocysteine levels or association with coronary artery disease. The largest study (n=4375) correlating MTHFR effects on venous thrombosis, by Bezemer, found no association with MTHFR 677 (7). These papers suggest that neither MTHFR 677 nor 1298 is a significant factor in patients who develop thromboses.
- Outside of vascular disorders, multiple studies have described associations of MTHFR variants with neoplastic diseases, neurologic abnormalities and developmental disorders. In addition to its role in folate and drug metabolism, the MTHFR enzyme functions in the methylation of DNA, RNA and proteins. As a result of enzymatic variants, reduced enzyme function may lead to overall reduced methylation. Moreover, enzymatic dysfunction may also lead to increased folate levels, which can be diverted to modify other metabolic pathways. Both factors, decreased methylation capacity and increased folate levels, either singly or in combination, have been associated with neoplastic disease. Homozygous 677T mutations have been associated with increased incidence of bilateral breast carcinoma, endometrial carcinoma and colorectal carcinomas (8).
- Folic acid supplementation is a critical component of early development. Women with decreased folic acid levels have increased occurrence and recurrence of neural tube defects in offspring. Some studies have shown MTHFR 677T homozygosity and combined 677/1298 heterozygosities have low folate status and an increased incidence of neural tube defects. Other congenital anomalies, such as cleft lip/palate, also have demonstrated positive associations with the 677T variant. MTHFR deficiency has also been associated with neurologic and psychiatric disease. Hyperhomocysteinemia has been found in patients with schizophrenia and depression. MTFHR polymorphisms can potentially alter the effects of any drug involved in folate transport and metabolism or in methylation reactions. These drugs include antifolates (methotrexate, 5-fluoruracil), anticonvulsants (Levodopa), hormone replacement medications, and lipid lowering drugs (fenofibrate or bezafibrate). When these drugs are administered, patients with the MTHFR 677T polymorphism have been found to have higher homocysteine levels. Moreover, with higher doses or when taken in combination, there can be increased toxicity (9).
How is diagnosis of MTHFR deficiency made?
As a screening method, the most common and cost effective laboratory test is quantitation of plasma homocysteine levels. These levels are known to fluctuate with environment including diet, underlying comorbidities, and interplay with other proteins associated with homocysteine and folate levels in vivo. Therefore, a definitive diagnosis requires MTHFR genotyping for common variants.
What are treatment implications for patient with MTHFR variants?
Patients with MTHFR gene polymorphisms such as 677T can lower homocysteine levels via folate supplementation. However, renal failure patients typically do not exhibit such responses (10). Patients with MTHFR gene variants and hyperhomocysteinemia may also have low magnesium levels. Therefore magnesium supplementation and low methionine intake may also benefit these patients.
Discussion with case patient's physician
MTHFR 677 and 1298 polymorphisms have an equivocal association with risk for venous thrombosis. Notably, the MTHFR 1298 polymorphism does not raise homocysteine levels nor confer an increased clotting risk as a single gene defect. Therefore, testing for the MTHFR 1298 variant does not seem reasonable given the homozygous wild type status at position 677 (677CC).
The 677T MTHFR polymorphism may be associated with mildly increased homocysteine levels, particularly if homozygous. However, it has been demonstrated that this variant is not associated with venous thrombosis risk in the heterozygous state. This patient had not had her homocysteine level measured; and based on her normal MTHFR 677 genotype, it is likely the homocysteine level is normal, and if mildly elevated might well respond to folate supplementation.
Given the clinical concern for hypercoagulability, it is appropriate to recommend the clinician consider testing instead for the relatively common and more pro-thrombotic factor V (F5) Leiden and prothrombin (factor II-F2) G202010A mutations. Moreover, a comprehensive panel of the more commonly inherited factors can be evaluated as a screening tool before the more expensive genetic testing. Specifically, protein S deficiency, protein C deficiency, activated protein c resistance, antithrombin deficiency and dysfibrinogenemia (11).
REFERENCES
- Narisawa K. Brain damage in the infantile type of 5,10-methylenetetrahydrofolate reductase deficiency. In: Botez MI, Reynolds EH, editors. Folic acid in neurology, psychiatry, and internal medicine. New York (NY): Raven Press, 1979: 391-400
- Erbe RW. Inborn errors of folate metabolism. In: Blakley RL, Whitehead VM, editors. Folates and pterines. Vol 3. Nutritional, pharmacological and physiological aspects. New York (NY): Wiley, 1986: 413-65
- Kluijtmans LA, van den Heuvel LP, Boers GH, Frosst P, Stevens EM, van Oost BA, den Heijer M, Trijbels FJ, Rozen R, Blom HJ. Molecular genetic analysis in mild hyperhomocysteinemia: a common mutation in the methylenetetrahydrofolate reductase gene is a genetic risk factor for cardiovascular disease. Am J Hum Genet. 1996 Jan;58(1):35-41.
- Gallagher PM, Meleady R, Shields DC, Tan KS, McMaster D, Rozen R, Evans A, Graham IM, Whitehead AS. Homocysteine and risk of premature coronary heart disease. Evidence for a common gene mutation. Circulation. 1996 Nov 1;94(9):2154-8.
- Jacovina AT, Deora AB, Ling Q, Broekman MJ, Almeida D, Greenberg CB, Marcus AJ, Smith JD, Hajjar KA. Homocysteine inhibits neoangiogenesis in mice through blockade of annexin A2-dependent fibrinolysis. J Clin Invest. 2009 Nov;119(11):3384-94. Epub 2009 Oct 19. Ishii H, Yoshida M, Hiraoka M, Hajjar KA, Tanaka A, Yasukochi Y, Numano F. Recombinant annexin II modulates impaired fibrinolytic activity in vitro and in rat carotid artery. Circ Res. 2001 Dec 7;89(12):1240-5.
- Lievers KJ, Boers GH, Verhoef P, den Heijer M, Kluijtmans LA, van der Put NM, Trijbels FJ, Blom HJ. A second common variant in the methylenetetrahydrofolate reductase (MTHFR) gene and its relationship to MTHFR enzyme activity, homocysteine, and cardiovascular disease risk. J Mol Med. 2001 Sep;79(9):522-8.
- Kupeli E, Verdi H, Simsek A, Atac FB, Eyuboglu FO. Genetic Mutations in Turkish Population With Pulmonary Embolism and Deep Venous Thrombosis. Clin Appl Thromb Hemost. 2010 Nov 15.
- Bezemer ID, Doggen CJ, Vos HL, Rosendaal FR. No association between the common MTHFR 677C->T polymorphism and venous thrombosis: results from the MEGA study. .Arch Intern Med. 2007;167(5):497-501.
- Ulrich CM, Kampman E, Bigler J, Schwartz SM, Chen C, Bostick R, Fosdick L, Beresford SA, Yasui Y, Potter JD. Colorectal adenomas and the C677T MTHFR polymorphism: evidence for gene-environment interaction? Cancer Epidemiol Biomarkers Prev. 1999 Aug;8(8):659-68.
- Schwahn B, Rozen R. Polymorphisms in the methylenetetrahydrofolate reductase gene: clinical consequences. Am J Pharmacogenomics. 2001;1(3):189-201.
Guenther BD, Sheppard CA, Tran P, Rozen R, Matthews RG, Ludwig ML.The structure and properties of methylenetetrahydrofolate reductase from Escherichia coli suggest how folate ameliorates human hyperhomocysteinemia. Nat Struct Biol. 1999 Apr;6(4):359-65.
- Bauer, KA. The thrombophilias; well-defined risk factors with uncertain therapeutic implications: Ann Intern Med 2001;135:367.
 Contributed by Lananh Nguyen, MD and Jeffrey Kant, MD, PhD
Contributed by Lananh Nguyen, MD and Jeffrey Kant, MD, PhD






![]() Contributed by Lananh Nguyen, MD and Jeffrey Kant, MD, PhD
Contributed by Lananh Nguyen, MD and Jeffrey Kant, MD, PhD