
FINAL DIAGNOSIS: RENAL INVOLVEMENT BY FABRY DISEASE
Fabry disease is an X-linked recessive inherited lysosomal storage disease, first described in 1898 by Johannes Fabry. It is an X-linked inborn error of the glycosphingolipid metabolic pathway, which results in accumulation of globotriaosylceramide (Gb3) in a variety of cells, including in the lysosomes. The metabolic defect in Fabry disease is deficiency of the lysosomal hydrolase alpha-galactosidase A (alpha-Gal A), which catalyzes the hydrolytic cleavage of the terminal galactose from Gb3. The alpha-Gal A protein is encoded by a 12-kb gene mapped to the long arm (Xq22.1 region) of the X chromosome. The condition affects hemizygous males, as well as both heterozygous and homozygous females. Males tend to experience the most severe clinical symptoms, while females vary from virtually no symptoms to those as serious as males. This variability is thought to be due to X-inactivation patterns during embryonic development of the female.
The prevalence of Fabry disease is estimated to range from 1:17,000 to 1:117,000 males in Caucasian populations. It is the second most prevalent lysosomal storage disorder after Gaucher disease. The disease is seen across all ethnic and racial groups. Symptoms include tinnitus, vertigo, nausea, diarrhea, hypohidrosis, fatigue, burning extremity pain, and skin changes. The typical skin lesion is a red, non-blanching papular angiokeratoma, which may appear in any region of the body, but are predominant on the thighs, buttocks, and lower abdomen. The patient in our case developed these angiokeratomas during pregnancy. This wide variety of symptoms and the rarity of Fabry disease can make initial diagnosis difficult.
Renal manifestations occur in approximately 50 percent of affected patients by the age of 35 years, and the incidence increases significantly with age. The early renal manifestations are a concentrating defect, with proteinuria appearing in adulthood, and renal failure developing by age 40-50 years. Thus, as in our case, proteinuria is often the first sign of renal involvement. Cardiac complications can also occur when Gb3 accumulates in myocytes. Cerebrovascular effects lead to an increased risk of stroke. Recall that our patient's father was also affected by Fabry disease, and died at a young age of 49 due to complications from cardiovascular and renal disease.
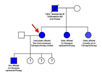
Until recently, the treatment of Fabry disease was limited to symptomatic palliative care. New enzyme replacement therapy at the cellular level has revolutionized treatment of Fabry disease. There are two drugs currently on the market, Agalsidase alpha (Replagal) and Agalsidase beta (Fabrazyme). The regimen requires intravenous infusion every two weeks, indefinitely. Unfortunately, the cost of these drugs can be prohibitive; treatment averages approximately $200,000 per year/per patient. This case highlights the interesting familial inheritance pattern of Fabry disease (Figure 17). Our patient has one sister who has opted to receive enzyme replacement therapy with Fabrazyme, and one sister who is not currently on therapy. Our patient also has a teenage son, who is more severely affected and has been on enzyme replacement with Fabrazyme for several years. She has three daughters who have not yet shown signs or symptoms of disease and have not been tested.
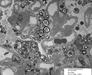
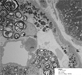
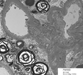
The percutaneous needle core biopsy of the kidney demonstrates diffuse renal involvement by Fabry disease, but without secondary chronicity changes at the present time. The classic light microscopy findings, well demonstrated in this case, are the prominently vacuolated glomerular visceral epithelial cells and tubular cells. The accumulation of Gb3 in the kidney is preferentially in glomeruli (podocytes) and the distal tubules. With progressive disease, mesangial expansion and glomerulosclerosis develop, with proportional interstitial fibrosis and tubular atrophy. Immunofluorescence microscopy may show IgM and C3 in mesangial areas. By electron microscopy, widespread deposits of Gb3 appear within enlarged secondary lysosomes as lamellated membrane structures, called myeloid or zebra bodies (Figures 9, 10, 11 and 12). These inclusions, composed of concentric layers with a periodicity of 3.5 to 5 nm and with an onion skin appearance, are considered a hallmark of glycolipid storage disorders. With the extent of renal involvement evident in this biopsy, the patient will likely consider initiating enzyme replacement therapy.
In conclusion, Fabry disease is an X-linked inborn error of the glycosphingolipid metabolic pathway. The accumulation of globotriaosylceramide in lysosomes leads to the manifestations of the disease, which can vary widely. In the setting of clearly established family history and classic phenotype, the diagnosis is usually confirmed by low leukocyte alpha-galactosidase activity. Renal manifestations occur in approximately 50% of affected patients by age 35, and the incidence increases significantly with age. If proteinuria and/or decreased kidney function develops, then a renal biopsy is helpful to evaluate for renal involvement. Characteristic findings by light microscopy include vacuolization of visceral glomerular epithelial cells (podocytes) and distal tubular epithelial cells. By electron microscopy, the lamellated myeloid or zebra bodies are considered a hallmark of glycolipid storage disorders. Once a diagnosis of Fabry disease is confirmed, there are new developments in cellular level therapy using enzyme replacement, which can significantly reduce the development of chronic disease. However, the duration of therapy is indefinite and the expense may be prohibitive for some patients.
REFERENCES:
![]() Contributed by Amber Henry, MD and Sheldon Bastacky, MD
Contributed by Amber Henry, MD and Sheldon Bastacky, MD