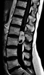
FINAL DIAGNOSIS: PARAGANGLIOMA WITH EXTENSIVE GANGLIOCYTIC DIFFERENTIATION.
DISCUSSION:
The predominant architectural feature is arrangement of the small cells in nests and lobules, or zellballen, which is a distinct characteristic of paragangliomas. Extensively hyalinized areas are present, which is not a classic feature of paraganglioma, but has previously been reported in these tumors [1,2]. Immunohistochemical stains for synaptophysin and neuron specific enolase highlight the chief cells, while the sustentacular cells show expression of S100. Mature ganglion cells are present, as well as cells that show features transitional between chief and ganglion cells. The ganglion cells express synaptophysin, neuron specific enolase, and neurofilament, indicating their neuronal differentiation. Glial fibrillary acidic protein is negative. The histologic features along with the staining pattern are diagnostic of a paraganglioma of the filum terminale with gangliocytic differentiation (or "gangliocytic paraganglioma").
Paragangliomas arise in specialized neural crest cells associated with segmental or collateral autonomic ganglia throughout the body [3,4]. Extra-adrenal paragangliomas occur most commonly in the head and neck region, usually involving the carotid bodies or glomus jugulare, and are observed less frequently in the mediastinum, retroperitoneum, lungs, duodenum, orbit, larynx, or urinary bladder [5]. Spinal cord paragangliomas are uncommon. Publication of the first case, though unrecognized at the time, has been attributed to Miller and Torak (1970), who described a "secretory ependymoma," [4,6,7]. In 1972, Lerman et al presented a "ganglioneuroma-paraganglioma of the intradural filum terminale" [2,8]. Since then, more than 120 CNS paragangliomas have been documented [4], and are most frequently found in the region of the cauda equine and filum terminale [1], although they have also been reported in the thoracic and cervical regions [2]. In a study of 30 spinal paraganglioma cases by Moran et al, 19 were located in the lumbar region, 6 in the cauda equina, 2 in the filum terminale, 2 in the thoracic region, and 1 in the cervical region [2]. In another study, 30 of 31 tumors originated in the cauda equina, and 25 of these arose from the filum [6].
One of the distinctive characteristics of our tumor is its gangliocytic differentiation. About 95% of gangliocytic paragangliomas are found in the second portion of the duodenum [9]. However, in a study of 31 cases of cauda equina paragangliomas, gangliocytic differentiation was seen in 45% of tumors [6]. In the study by Moran et al, only one of thirty spinal paragangliomas showed ganglion cells [2].
The typical clinical presentation consists of lower back pain, usually accompanied by sciatica [2,10], as was seen in our patient. In a study involving 31 patients with cauda equina paraganglioma [6], 11 patients (35%) experienced motor or sensory deficits involving the lower extremities; 5 (13%) had urinary/fecal incontinence; and 2 (6%) were paraplegic at presentation. Hydrocephalus has also been reported [11]. Patients range in age from 13 to 71 [3], and in Moran's study, the median age was 46 years [2]. Sonneland's series showed a male predominance of 1.4:1 [6].
On MRI, the tumors are generally lobulated or ellipsoid, encapsulated, hypo- or isointense to the spinal cord on T1-weighted images, hyperintense on T2-weighted images, and contrast-enhancing, as shown below (Image 21) [3,10]. Radiologicallly, paraganglioma is impossible to distinguish from myxopapillary ependymoma, which is the main tumor in the differential diagnosis [10].
Based on the clinical, radiologic, and gross intraoperative findings, the postoperative diagnosis in our case was reported as ependymoma. This is not surprising since myxopapillary ependymoma is the most common neoplasm found in this region [10,12], and differentiation between ependymoma and paraganglioma requires histologic evaluation. Although microscopic examination of our tumor revealed some areas in which cells appeared to form perivascular pseudorosettes (Image 5) and other areas with extensive hyalinization (Image 7) similar to that seen in myxopapillary ependymomas, the majority of our tumor exhibited classic zellballen architecture along with mature ganglion cells, which are not usually seen in ependymomas [4]. Therefore, in our case, morphology was most suggestive of a paraganglioma. However, if the "classical patterns" associated with paraganglioma and ependymoma are not present, it can be quite difficult to distinguish between the two. In these cases, differentiation can usually be accomplished based on the pattern of immunohistochemical staining. Most paragangliomas exhibit positivity with immunostains to neuron-specific enolase, synaptophysin, chromogranin, and neurofilament, while glial fibrillary acidic protein (GFAP) usually stains only the sustentacular cells. Conversely, the vast majority of ependymomas show expression of GFAP, while neuroendocrine markers are negative [3,13]. Ultrastructurally, paragangliomas are characterized by numerous perinuclear dense-core granules, as shown below (Image 22).
Although cauda equina paragangliomas are categorized as a WHO grade 1 [3], and are usually well-demarcated, encapsulated, and slow-growing [6,11], locally invasive and recurrent tumors have been reported [10]. In the study by Moran et al, follow up information was available for 20 patients, none of whom received postoperative irradiation [2]. Of these, 18 were alive without disease (6 to 216 months post-surgical) and one had developed metastases to bone. Two patients had died of unrelated causes. Overall, the recurrence rate is estimated at 4% after total resection [3]. Foci of hemorrhagic necrosis, scattered mitotic figures, and nuclear pleomorphism may be observed, and none of these features are clinically significant [3].
In summary, paragangliomas (particularly those with gangliocytic differentiation) are uncommon tumors of the spinal cord and are usually confined to the cauda equina region. In most cases, they behave in a benign fashion and complete surgical resection may be curative. Irradiation or chemotherapy may be used if the tumor cannot be entirely removed, although chemotherapy has not shown consistent benefit [7]. Because ependymal tumors are more frequent in this region, a paraganglioma may be overlooked, and it is important to include this entity in the differential diagnosis.
REFERENCES:
![]() Contributed by Siobhan O’Connor, MD and Kate McFadden, MD
Contributed by Siobhan O’Connor, MD and Kate McFadden, MD