FINAL DIAGNOSIS: Granulomatous Amoebic Encephalitis due to Acanthamoeba castellanii
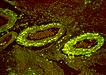
With immunofluorescent antibody techniques, the amoebae showed distinct fluorescence with anti-A. castellanii at 1:20 and 1:50 dilutions (Image 09), but they were negative or weakly positive with anti-A. culbertsoni, A. polyphaga, A. rhysodes, and A. astronyxis sera. Protozoa were also seen in autopsy lung tissue, and identified as A. castellanii. There was also necrotizing amoebic panniculitis in subcutaneous, peripancreatic, mesenteric and peri-aortic tissue. There were occasional amoebae in the liver, but not enough to account for all of the patient's liver disease, so part of his liver disease was most likely due to sepsis due to his disseminated amoebiasis.
Contributors' Notes
Granulomatous amoebic encephalitis is a rare subacute necrotizing infection which usually occurs in chronically ill, debilitated patients, particularly those who are immunosuppressed or immunodeficient.(1) Granulomatous amoebic encephalitis is usually manifested by the insidious onset of headache, low-grade fever, mood swings, lethargy and confusion. Seizures, focal neurological deficits (such as hemiparesis or cranial neuropathy), signs of meningeal irritation (such as stiff neck) and increased intracranial pressure (manifested by nausea and vomiting) are common.(1) Computerized tomography (CT) scans may demonstrate bilateral low-density areas with mild mass effect in the cortex and subcortical white matter, and magnetic resonance imaging (MRI) scans may show increased signal on T2-weighted images.(2) The lesions may show ring enhancement with intravenous contrast studies. Occasionally, there are neuroradiographic findings of an expanding intracranial mass that may mimic a cerebral tumor or a brain abscess. The cerebrospinal fluid usually shows a lymphocytic pleocytosis, with mildly elevated protein and normal glucose, but diagnostic organisms are not readily identified in the cerebrospinal fluid and lumbar puncture is contraindicated in many of these patients because of their increased intracranial pressure.(1,3) Infection of the central nervous system is thought to be hematogenous, from sites of primary infection in the lungs or the skin. The patient in this case had Acanthamoeba infection of both skin and lungs, in addition to the brain, at the time of death, and it is difficult to know which might have been the primary site of infection.
Granulomatous amoebic encephalitis is most commonly caused by Acanthamoeba castellanii, A. culbertsoni, A. polyphaga or Balamuthia mandrillaris.(1) It is rarely due to Entamoeba histolytica. E. histolytica rarely infects the central nervous system and when it does, it tends to cause an abscess with a fulminant clinical course culminating in the patient's death within 12-72 hours (untreated). E. histolytica infection of the brain also tends to occur in patients with a previous diagnosis of E. histolytica infection of the intestines, the liver or the lungs. Granulomatous amoebic encephalitis is also rarely due to Naegleria fowleri. N. fowleri generally causes acute encephalitis in immunocompetent hosts who go swimming underwater or diving outdoors in fresh water in warm weather. Chronically ill, debilitated, immunosuppressed or immunodeficient patients tend not to engage in such activities.
The pathogenicity of the three Acanthamoeba species associated with granulomatous amoebic encephalitis, A. culbertsoni, A. castellanii and A. polyphaga, has recently been linked to their ability to activate the alternative complement pathway yet resist complement-mediated cellular lysis.(4) All three of these Acanthamoeba species depleted hemolytic complement activity from normal human serum, yet were resistant to its lytic effects. This appeared to be due to both the release of a transport-dependent extracellular matrix as well as the presence of complement inhibitory surface proteins. It is intriguing that the patient in this case had Legionella pneumonia only 3 weeks after transplantation and immunosuppression, because Legionella is an intracellular pathogen of humans that is amplified in the environment by intracellular multiplication within protozoa.(5) Within both macrophages and protozoa, the bacterium multiplies in a rough endoplasmic reticulum-surrounded phagosome that is retarded from maturation through the endosomal-lysosomal degradation pathway. Preadaptation of Legionella to infection of protozoa may play a major role in its ability to replicate in human cells and cause Legionnaires' disease. It seems quite possible that this patient may have acquired Acanthamoeba at the same time he acquired Legionella. Smoldering amoebic infection could account for much of the illness he experienced in the 28 months following transplantation. Acanthamoeba may have caused his facial abscess, his sinusitis and possibly even his diarrheal illness and urinary tract infection.
The gross pathology of granulomatous amoebic encephalitis is characterized by multifocal encephalomalacia, edema, necrosis, hemorrhage and sometimes abscess formation.(1) The meninges overlying these foci may be cloudy. There may be uncal or cerebellar tonsillar herniation. Lesions occur in the cerebral hemispheres, the basal ganglia, the brainstem and the cerebellum. Microscopically, the characteristic lesion is a necrotizing subacute or chronic granulomatous encephalitis with lymphocytes, macrophages and multinucleated giant cells, associated with variable numbers of organisms. There may be thrombosis of small blood vessels associated with necrosis and hemorrhage. In patients with the acquired immuno-deficiency syndrome (AIDS), the inflammatory reaction is typically scarce and composed mainly of CD-68 positive macrophages.(3)
The amoebae producing granulomatous encephalitis characteristically produce cysts in the infected tissue whereas E. histolytica and N. fowleri do not.(6) The cysts of Acanthamoebae are rounded, 10-15 microns in diameter and have a thick, double-contoured, wrinkled wall. The cysts of Balamuthia are larger, 15-30 microns in diameter and have a thicker wall and more globular refractile contents than the cysts of Acanthamoebae. The trophozoites of all the amoebae have an irregular amoeboid shape. The trophozoites of Acanthamoebae tend to be 8-12 microns in greatest dimension in tissue sections. The cytoplasm is highly vacuolated and the nucleus has a large round dense karyosome with a halo around it. The trophozoites of Balamuthia are larger, 15-35 microns in greatest dimension, with a less prominent nuclear karyosome. The amoebaee which cause granulomatous encephalitis can be identified in tissue sections stained with routine hematoxylin and eosin (H&E), but they can be also be seen with methenamine silver, periodic acid-Schiff (PAS) and Masson trichrome stains. They are difficult to identify with Gram stain.(7)
Acanthamoebaee can be cultured on blood or chocolate agar plates, producing tracks in the agar surface, but these tracks are small and difficult to recognize.(7) Monoxenic culture on 1.5% nonnutrient agar preseeded with a lawn of Escherichia coli usually yields growth in 2-3 days, but identification and differentiation of the pathogenic and non-pathogenic strains is not easy.(8) Morphological criteria are inadequate, while thermophilic character, pH dependency and even virulence in infected mice are not unambiguous features of pathogenicity of the different strains. Molecular methods, employing polymerase chain reaction nucleic acid testing, restriction endonuclease digestion of whole-cell or mitochondrial DNA, or isoenzyme profile analysis after isoelectric focusing and staining for acid phosphatase and propionyl esterase activity, are more sensitive and specific, but also far more laborious and expensive.(8) Monoclonal antibodies are also available for immunofluorescence microscopy testing, as illustrated in this case, but it is not practical for most hospital laboratories to keep these reagents, which would usually outdate before they were used, and small hospital laboratories may not have no facility for fluorescence microscopy.
Brain biopsies are commonly diagnostic. Direct identification or isolation of the protozoa from the central nervous system provides a specific diagnosis. Granulomatous amebic encephalitis usually has a prolonged clinical course and early diagnosis by brain biopsy holds the hope of successful treatment because there are multiple drugs, such as ketoconazole, miconazole, 5-flucytosine and pentamidine, effective against the causative organisms in vitro.
REFERENCES
![]() Contributed by Jiangzhou Wang, MD, PhD, Larry Nichols, MD and A. Julio Martinez, MD
Contributed by Jiangzhou Wang, MD, PhD, Larry Nichols, MD and A. Julio Martinez, MD