Jacquot C et al. Pediatr Blood Cancer. 2021; 68: e29082
FINAL DIAGNOSIS
Red Blood Cell T-activation due to Invasive Pneumococcal Disease
DISCUSSION
Background and Pathogenesis
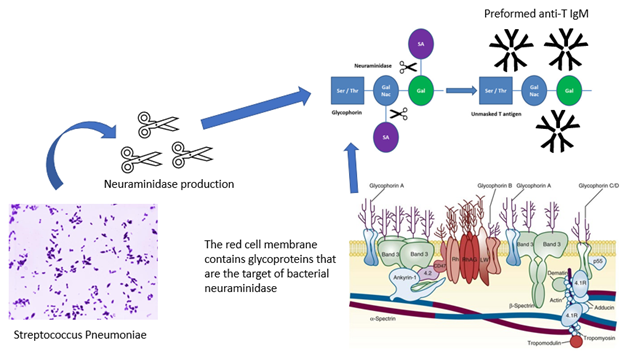
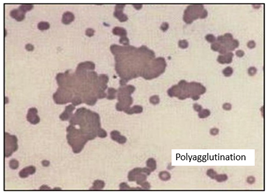
T-activation is one cause of red blood cell (RBC) polyagglutination. "Polyagglutination" refers to a phenomenon whereby glycoproteins present on the RBC surface are altered, and consequently, the RBCs agglutinate in the presence of otherwise ABO compatible adult sera/plasma. Alteration of RBC membrane proteins occurs because of bacterial or viral infection, or an inherited condition, such as Cad, hemoglobin M-Hyde Park, hereditary erythroblastic multinuclearity with a positive acidified serum (HEMPAS), or NOR.1
Although rare in general, polyagglutination most often occurs due to microbial infection. In bacterial induced-polyagglutination, enzymes produced by the bacteria cleave carbohydrate groups present on the RBC surface glycoprotein and reveal "hidden" antigens or "cryptantigens." 1,2 Streptococcus pneumoniae is a prototypical example. S pneumoniae produces a neuraminidase, which cleaves N-acetyleneuraminic acid residues from glycoproteins and exposes a beta-linked galactosyl residue (Gal-b(1-3)-GalNAc), or the Thomsen (Tclassical) receptor3. Once exposed, the cryptantigen becomes the target of pre-formed anti-T antibodies present in most adult human sera/plasma (see below). Anti-T antibodies are typically of IgM subclass and may form following exposure to bacterial antigens that appear "T-like" in structure while in infancy. Most infants do not have detectable anti-T IgM until 2 to 6 months of age, but nearly all children have adult levels by 2 to 5 years1,2,4,5.

Jacquot C et al. Pediatr Blood Cancer. 2021; 68: e29082
|
Detectable polyagglutination occurs with extensive (50 - 70%) cryptantigen exposure5. This level of exposure may occur with septicemia or leakage of enzymes from an extravascular infection1. Different bacteria may produce enzymes that cleave RBC glycoproteins at different locations and therefore, produce variable in vivo polyagglutination and clinical phenotypes. Other examples of T-activation are listed in the table below. Not all patients with T activation express the Tclassical receptor. Instead, many express T receptor variants (Th, Tk, Tx). This is significant because naturally occurring anti-T IgM targets the Tclassical receptor, not other types2,6. |
| Photo credit: https://www.researchgate.net/publication/49756140/ |
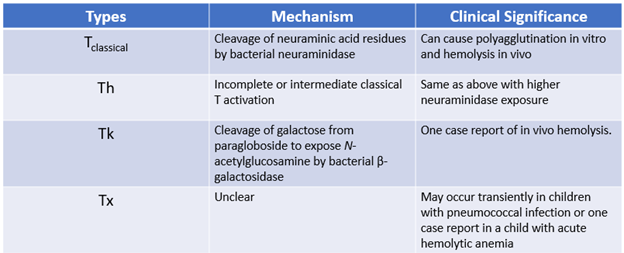
When in vivo polyagglutination occurs, the patient may experience hemolysis; however, few cases of hemolysis definitively linked to T activation have been reported2,4. The anti-T antibody theorized to cause hemolysis is of IgM subclass and binds most strongly at 4°. Weak binding may occur at body temperature, 37°, but it is unclear if this would be sufficient to explain the hemolysis seen in critically ill patients. Instead, mechanical shearing of red cells due to bacterial endotoxin-induced disseminated intravascular coagulation or iatrogenic sources such as continuous renal-replacement therapy may play a larger role.
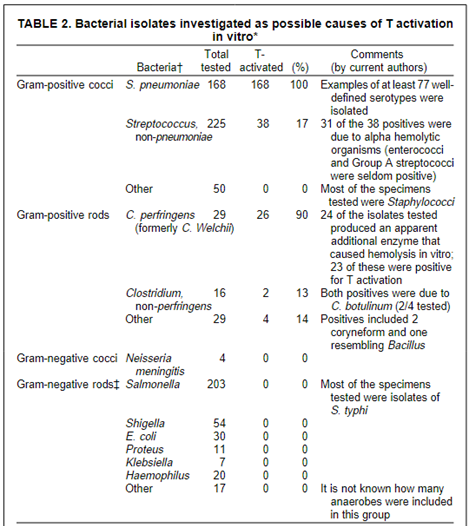
T-activation is typically an acquired, transient phenomenon. Because detection requires specific testing (described below), a diagnosis of T activation may be missed if not suspected. T-activation is seen most often in children and infants, who have been infected with Clostridium perfringens, Streptococcus pneumoniae, and influenza, though other organisms have been implicated (see table below)6. The most common associated clinical syndromes are necrotizing enterocolitis and atypical hemolytic uremic syndrome2,4. However, it should be mentioned that T-activation has also been reported in healthy children and blood donors1,7.
Necrotizing Enterocolitis (NEC)
T activation has been reported in 5 - 35% of neonates with NEC and may correspond with worsened clinical severity2,8. The clinical ramifications of T activation in infants with NEC are unclear. In the largest retrospective study of T activation to date, Boralessa et al screened 375 infants admitted to a neonatal intensive care unit for T and T variant activation. Forty-eight of 375 (12.8%) had evidence of T activation.
Crookston KP et al. Transfusion. 2002. 40(7): 801-12
Of those 48, nine (19%) developed NEC during their hospitalization, though detection of the T activation was not always temporarily associated with onset of NEC. Furthermore, no infant who received plasma-containing (i.e., anti-T containing) blood products developed hemolysis7. The use of washed blood products (discussed below) in this condition remains controversial.
Atypical Hemolytic Uremic Syndrome (HUS)
Several small cases series have demonstrated a high incidence (60 - 100%) of T activation in invasive pneumococcal disease (IPD)9-12. T activation appears to peak 5 - 10 days following the onset of symptoms. Initial detection of T activation in patients who present with pneumococcal disease may serve as a harbinger of worsened disease severity. Huang et al demonstrated that positive T activation was 86% sensitivity and 57% specific for severe manifestations of pneumococcal disease (pneumococcal HUS and hemolytic anemia) in a retrospective cohort.13 Furthermore, Chen et al found a longer duration of anemia and thrombocytopenia (3.4 vs 0.92 days, p = 0.03) and greater incidence of acute kidney injury (52 vs 15%, p = 0.028) in T activated patients with IPD compared to non-T activated patients10. However, correlation is not equivalent to causation. Many questions remain unanswered regarding the role T activation, anti-T IgM antibodies, complementation activation, and neuraminidase play in the pathophysiology of HUS.
Laboratory detection of T activation
Historical testing
Prior to use of monoclonal reagents, T activation might be detected during routine ABO typing or direct antiglobulin testing (DAT). Human-derived reagents contain anti-T IgM antibodies that can agglutinate red cells expressing cryptantigens, causing ABO discrepancies in the case of ABO typing or false-positive DAT results. Monoclonal testing reagents, which lack naturally occurring anti-T IgM antibodies, have largely replaced human-derived reagents, and therefore, polyagglutination is not detected during routine ABO typing. Of note, this is also the reason the (frequently tested on board exams) acquired B phenomenon is no longer detected. DAT may detect red cells heavily coated with anti-T IgM antibodies1,14.
Modern testing
Minor Crosshatches
When a patient is suspected to have T activation, a sample from the patient is first subjected to several minor crossmatches. A minor crossmatch is performed by mixing donor plasma with the patient’s red cells at immediate spin and observing for agglutination. Minor crossmatches should be performed with plasma from several healthy, non-alloimmunized adult donors and cord samples. For the latter, the maternal samples must be devoid of alloantibodies. The T-activated patient should demonstrate agglutination to most if not all adult donor plasma but no cord plasma, as neonates have not had the opportunity to develop anti-T IgM antibodies and maternal IgM antibodies will not cross the placenta. Adults express variable levels of anti-T IgM, so several adult AB plasma samples should be tested. Because anti-T IgM antibodies are unstable, fresh plasma should be used for testing whenever possible. The autocontrol (testing of the patient’s serum to the patient's red cells) is expected to be negative. In patients with T activation, the naturally occurring anti-T antibodies are absorbed out of the serum by red cells and other tissues expressing the T antigen1.
Lectins
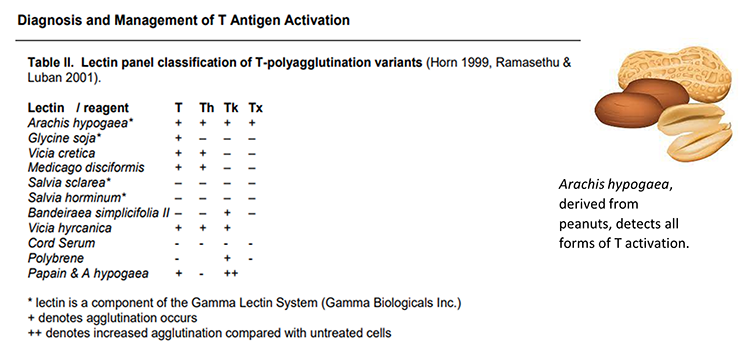
Lectin testing is the most effective means of diagnosing T activation. Lectins are proteins derived from plants and invertebrate animals which can bind to specific RBC antigens. Highly concentrated lectin reagents may be pan-reactive to all RBCs; however, a panel of carefully prepared lectin reagents may distinguish different types of T activation. See table below for all lectins used in polyagglutination testing1,2.
Other testing
Several other studies may help diagnose T activation and/or polyagglutination in at-risk patients. The DAT may be positive for IgG and C3d in patients with extensive T activation. However, several real world studies have shown variable DAT positivity in patients with positive lectin studies4,7. The patient should also be evaluated for evidence of hemolysis with haptoglobin, lactate dehydrogenase, fractionated bilirubin levels, reticulocyte count, urinalysis, and plasma free hemoglobin (if available). Peripheral smear evaluation may demonstrate agglutinated RBCs in vitro.
Blood bank management
Blood products
Transfusion of plasma-containing blood products to patients demonstrating T activation is controversial. As mentioned above, few cases of transfusion-associated hemolysis in patients with T activation have been reported. However, these patients are critically ill with many possible etiologies for hemolysis; therefore, it is difficult to determine if anti-T antibodies play a definitive, clinically significant role in the hemolysis observed in patients with invasive microbial infections. Conservative clinicians avoid transfusing plasma-containing products to clinically stable, T activated patients with anemia, thrombocytopenia, or coagulopathy without bleeding, or, if transfusions are necessary, provide products that are expected to contain little, if any anti-T IgM3. These products include washed red cells, platelets suspended in platelet additive solution, low titer anti-T fresh frozen plasma, fractionated blood products (e.g., three or four factor complex concentrate, albumin, and IVIG), and recombinant factor concentrates (e.g., rVIIa)2.
Not all patients who demonstrate T activation experience in vivo polyagglutination or hemolysis; therefore, some clinicians adopt a more reactive strategy, where patients who develop a pattern of hemolysis following transfusion receive low anti-T containing products. These products may be especially important when patients require large quantities of blood products, such as during a massive transfusion, multiple blood products over several days, or plasma exchange. For coagulopathic patients, use of supplemental vitamin K, factor concentrates, and recombinant products may be beneficial2.
Prior to adoption of either strategy, patients must demonstrate Tclassical activation on lectin testing. As mentioned previously, patients who are found to have T variant activation (i.e., T activation other than Tclassical) may not require plasma avoidance because most donor plasma does not contain antibodies to other T receptors2,7.
Testing
Patients with T activation should be screened weekly for continued T activation. Once T activation testing is negative, the patient may receive standard blood component therapy.
CASE RESOLUTION
Work up was consistent with a diagnosis of Tclassical activation in this patient with pneumococcal hemolytic uremic syndrome. The patient developed hemolysis and clinical deterioration following her first RBC transfusion; therefore, she was placed on a washed RBC and platelet protocol. She received antibiotics and supportive care in the pediatric intensive care unit, and over time showed clinical improvement.
Ten days following initial T activation testing, she was tested again, and results were as follows:

Given resolution of her T activation, the washed protocol was discontinued, and the patient received standard blood products uneventfully. She was ultimately discharged to begin rehabilitation after a prolonged hospitalization.
MULTIPLE CHOICE QUESTIONS
1. RBC polyagglutination results from cryptantigens on the red cell surface. What are cryptantigens?
2. Most adults express anti-T antibodies in their sera/plasma. These anti-T antibodies typically have specificity to which type of T activation?
3. Which two clinical syndromes are most often associated with T activation in children?
4. Identification of T activation requires testing with lectins (proteins derived from plants and invertebrates that have specificity to RBC surface antigens). Which lectin binds to all forms of T activation?
5. If you wished to avoid giving plasma-containing blood products to a patient with T activation, which would be acceptable to give?
REFERENCES
![]() Contributed by Jennifer M. Jones, MD and Lirong Qu, MD
Contributed by Jennifer M. Jones, MD and Lirong Qu, MD