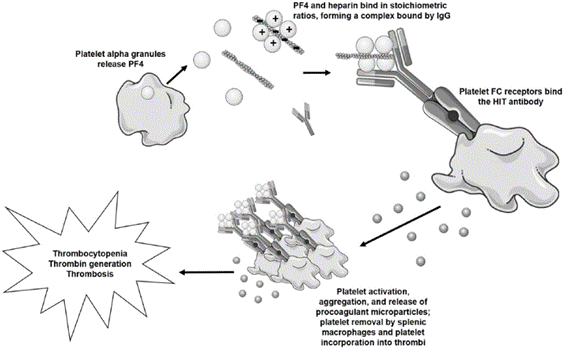
Figure 1. Hogan et al; Vascular Medicine, 2020.
FINAL DIAGNOSIS
Presumptive Heparin Induced Thrombocytopenia (Type 2)
DISCUSSION
Background and Pathogenesis
Heparin induced thrombocytopenia (HIT) is a potentially severe complication that occurs in approximately 5% of patient that are exposed to unfractionated heparin (UFH). The risk is lower (~1%) with low molecular weight heparins (LMWH). HIT is a clinical-pathological syndrome and diagnosis requires a characteristic clinical presentation and laboratory evidence of the pathogenic "HIT antibodies". Clinically, it is characterized by a fall in platelet counts approximately 5-14 days after heparin exposure and a paradoxical hypercoagulable state. Up to 50% of patients with HIT develop thrombotic complications with approximately 30% mortality and morbidity. There are two types of HIT. Type 1 HIT is more common and non-immune mediated. The thrombocytopenia is typically mild, transient, and occurs 1-3 days after exposure. It is not a contraindication to further heparin use. Conversely, type 2 HIT is immune mediated, and occurs 5-14 days after exposure. The thrombocytopenia can be severe. Future heparin products are contraindicated in patients with a history of type 2 HIT. In the acute phase alternative anticoagulation must be initiated to prevent thrombotic complications.
The pathogenesis of HIT is thought to be due to platelet factor 4 (PF4). PF4 is stored in alpha granules of platelets and released upon platelet activation. It is positively charged, and thus can bind negatively charged heparin. This can trigger the formation of IgG, IgA, or IgM antibodies against the PF4/heparin complex (Figure 1). These IgG antibodies can in turn bind to the antibody FC receptor on platelets, either activating them (increasing the risk of thrombosis) or tagging them for removal by the reticuloendothelial system (causing thrombocytopenia).

Figure 1. Hogan et al; Vascular Medicine, 2020.
Diagnosis As HIT is a clinicopathologic diagnosis, the first step is to evaluate the patient clinically with the 4 T's scoring system proposed by Lo et al. (Figure 2). Patients with a low score of <3 are unlikely to have HIT and do not need further testing and can restart/continue to receive heparin if clinically indicated. Patients with intermediate or high 4 T's scores (≥4) should undergo initial screening with an ELISA assay to detect antibodies against heparin platelet factor 4 (HPF4). This assay is often reported as an optical density (OD), signifying the strength of the reaction. The OD values are used to assign cutoffs that can change across laboratories and manufacturers. This assay is very sensitive and if the HPF4 assay is negative, HIT is unlikely and essentially ruled out. If the OD is low or intermediate positive, a confirmatory functional assay such as a serotonin release assay (SRA) (gold standard) or heparin-induced platelet aggregation (HIPA) assay should be used to confirm the diagnosis (Figure 3). If the optical density is significantly elevated, confirmatory testing can be bypassed as the probability of HIT increases as the OD increases.
While SRA assays are the current gold standard, they are laborious, require radioactive serotonin particles, and are increasingly offered at few specialized reference laboratories. Flow cytometry for P-selectin is simpler to perform and shows promise for diagnosing HIT but is not in widespread use to date.
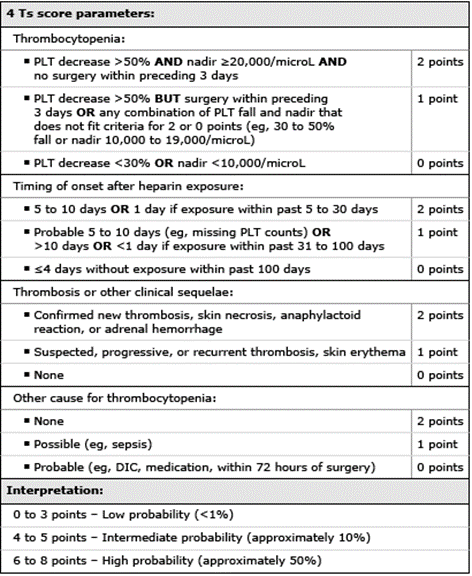
Figure 2. 4 T's score. UpToDate, "Clinical presentation and diagnosis of heparin-induced thrombocytopenia".
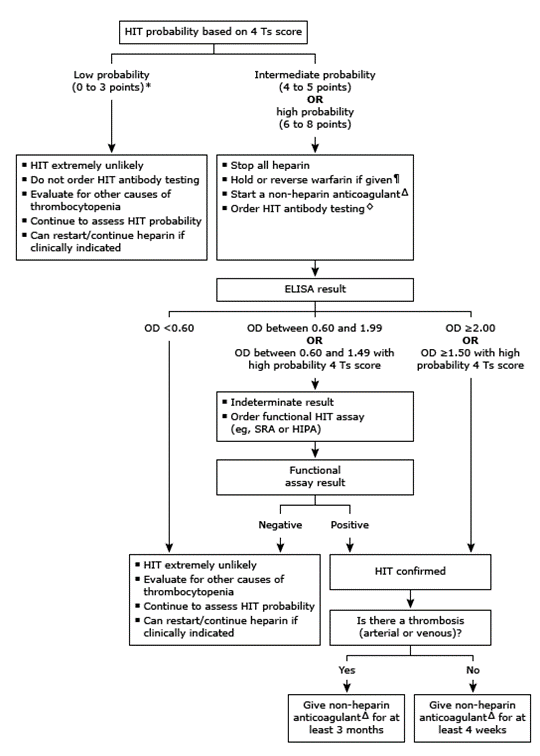
Figure 3. Algorithm for HIT evaluation. UpToDate, "Clinical presentation and diagnosis of heparin-induced thrombocytopenia".
Laboratory testing for HIT
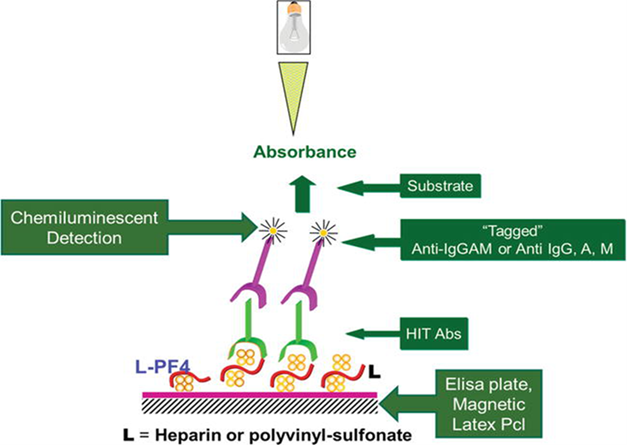
Figure 4. Amiral et al., Anticoagulation Drugs - the Current State of the Art; 2019.

Figure 5. Warkentin TE, Hematology Am Soc Hematol Educ Program; 2011.
Serotonin release assay (SRA) (gold standard)- measures the release of radiolabeled serotonin from dense granules when platelets are activated.
Normal donor platelets "loaded" with radioactive serotonin are mixed with patient's serum containing antibodies. Heparin is added at different concentrations. Maximum serotonin release occurs at 0.1-0.3 IU/ml of heparin concentration. Serotonin release is extinguished at supratherapeutic heparin concentrations. HIT is diagnosed when serotonin is released from platelet dense granules at low heparin concentrations. Although many laboratories consider SRA to be positive at 20% release of serotonin, typically 50-80% of serotonin release is observed at low heparin concentrations.
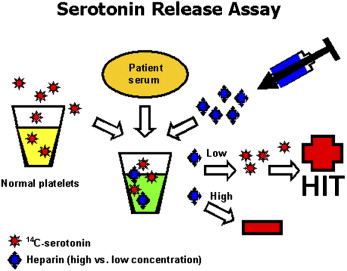
Figure 6. Baldwin et al., Surgery; 2008.
This assay is performed by mixing patient's serum with donor platelets in the presence of heparin. Aggregation of the donor platelets indicates the presence of antibodies to the heparin-PF4 complex. Heparin is added to the mixture at the different concentrations and is visually monitored for aggregation. If aggregation occurs, a higher concentration of heparin is added to determine a point at which aggregation is inhibited. Results are classified as negative or positive, depending on the concentration of heparin required to reduce the aggregation observed. HIPA is very specific, but less sensitive test compared to SRA.
In the presented case, HPF4 ELISA and SRA were ordered simultaneously due to the acute need for cardiac transplantation and availability of a donor heart. The initial round of testing revealed interesting results. The HPF4 ELISA was negative (OD 0.28). Conversely, the confirmatory SRA was weakly positive (<50% serotonin release at the lowest heparin concentration) but displayed the classical pattern seen in HIT patients. In their summary paper in the American Journal of Hematology, Warkentin et al show that cases of EIA negative but SRA positive HIT patients like the presented case are rare but possible.
Given the clinical uncertainty and acute need for surgery, a repeat round of testing was ordered. The second EIA was weakly positive (OD 0.65) while the repeat SRA was negative. Some studies have demonstrated that SRA may become negative once platelet counts normalize as in this case. The patient was given a diagnosis of presumptive HIT due to his weakly positive initial SRA and improvement in platelet counts with a non-heparin anticoagulant.
Cessation of all heparin products is the first step to managing HIT. This prevents further antibody generation. Patients are still at risk of thrombosis for the next 4-5 days. Any non-heparin anticoagulant can be used in its place, with the exception of warfarin. Warfarin is avoided in the short term due its initial hypercoagulable state via inhibition of protein C and protein S, which have short half-lives. If the patient was on warfarin at the time of HIT diagnosis, reversal with vitamin K is recommended. Common treatment modalities include direct thrombin inhibitors or factor Xa inhibitors. In the presented case, the patient's platelet counts improved after switching to fondaparinux. The duration of anticoagulation depends on if a thrombotic event occurred. Evaluation for thrombosis is also important in patients with HIT. Bilateral lower extremity duplex ultrasounds are recommended given that 50% of patients have some form of thrombosis at the time of diagnosis. There is no role for anti-thrombolytics in the setting of HIT. Platelet transfusions are relatively contraindicated as they can increase the risk of thrombosis in patients with acute HIT.
For patients with HIT, future heparin administration should be avoided when at all possible. However, heparin remains the anticoagulant of choice for patients undergoing cardiac and vascular surgeries because of its ease of monitoring, favorable pharmacokinetics, reversibility with protamine sulfate and familiarity to clinicians. For patients with who have been diagnosed with HIT and have received heparin more than six months ago, heparin can be used safely for cardiac surgery since HIT antibodies usually disappear by that time. Thus, if a surgery is non-emergent it is best to wait for antibodies to disappear. If the diagnosis of HIT was less than 6 months ago, immunologic assays may be ordered, and if negative, heparin can also be used during cardiac surgery.
However, patients who need urgent or emergent cardiac surgery and have positive HPF4 antibodies require different approach. In these patients, preoperative apheresis can be used to remove circulating antibodies. A 1 or 1.5 liter volume exchange with FFP can remove 85% to 100% of the circulating antibodies. Apheresis can be done the day prior to surgery or in the operating room prior to heparin administration (Current Category 3, Grade 2C AFSA recommendation). In the presented case, the patient underwent one round of plasma exchange the day before the operation in conjunction with another round intraoperatively. This was performed on our presumed HIT-positive patient due to the acute need of cardiac transplantation and availability of a donor heart. The stakes are high surrounding cardiac transplantation. Failure to properly anticoagulate in this setting could result in loss of graft or patient mortality.
In contrast, a direct thrombin inhibitor (DTI), bivalirudin, can be used for cardiopulmonary bypass (CPB) in patients with acute HIT. If the surgery is done "off-pump" either bivalirudin or argatroban can be used. Apheresis is recommended to decrease the level of DTI post CPB if excessive bleeding occurs. In all HIT patients who require anticoagulation post operatively a DTI (argatroban or bivalirudin) should be used for anticoagulation. A final option is to administer high dose IVIG to prevent removal of platelets by the reticuloendothelial system.
MULTIPLE CHOICE QUESTIONS
1. Which is part of the "4 T's" scoring method for assessing HIT clinically?
2. What is the main antibody implicated in the pathogenesis of HIT?
3. Which assay can be used as an alternate confirmatory test instead of the serotonin release assay?
4. When managing suspected HIT, what is the best first step?
5. If a patient with HIT requires an emergent surgical procedure where heparin is to be given (such as cardiac transplantation), which of the following is a viable treatment strategy?
REFERENCES
![]() Contributed by Mason Marshall, DO and Irina Chibisov, MD
Contributed by Mason Marshall, DO and Irina Chibisov, MD