
![]() Pooja Srivastava MD, Svetlana A. Yatsenko, MD, DABMGG, FACMG
Pooja Srivastava MD, Svetlana A. Yatsenko, MD, DABMGG, FACMG
INTRODUCTION
Acute myeloid leukemia (AML) is a highly aggressive and extensively studied hematological malignancy which is known to affect around 13000 adults in United States annually (1). Cytogenetic aberrations are the most important diagnostic and prognostic findings in patients with AML, however karyotype abnormalities are identified in only half of all AML cases (2, 3). Cryptic rearrangements involving the lysine (K)-specific methyltransferase 2A (KMT2A) has been detected in ~ 5-10% cases of adult patients with cytogenetically normal AML (4, 5). The KMT2A gene, located at 11q23, is involved in a number of the translocations with more than 90 gene partners. Many of these rearrangements can be detected by karyotype or by fluorescence in situ hybridization (FISH) studies using KMT2A break apart probe. A partial tandem duplication (PTD) in the KMT2A gene is a cryptic alteration, undetectable by either karyotype or FISH, and produces an in-frame, elongated protein (6). We present a case of AML with normal cytogenetic results, in which a KMT2A-PTD was detected by microarray analysis.
CASE REPORT
A 63-year-old female with no past medical history came for routine general physical check-up. Her complete blood count (CBC) analysis was consistent with neutropenia and thrombocytopenia. Her white blood count (WBC) was 0.8X109/L, absolute neutrophil count (ANC) 0.2X109/L, absolute lymphocyte count 0.6X109/L, hemoglobin 12.7g/dl, platelet count 118X109/L, MCV 92fl, creatinine 0.8mg/dl, and normal LFTs. Her previous CBC from 2 years back was normal with WBC of 4.1X109/L, ANC 2.4X109/L, absolute lymphocyte count was slightly decreased at 1.2X109/L, hemoglobin 14.4g/dl, MCV 88fl, and platelet count 204X109/L. Clinically the patient was asymptomatic. On further questioning she acknowledged having some mild sinus congestion, but no fevers, chills, or pruritus. On examination she was found to have some bruising but the exam was negative for palpable lymphadenopathy or splenomegaly.
Flow cytometric analysis was performed on a peripheral blood sample and revealed a small circulating blast population (<1%) which was considered non-specific. Due to the concerns for a bone marrow infiltrating process, a bone marrow aspirate and biopsy were performed. Also, serum protein electrophoresis, serum protein immunoelectrophoresis, and quantitative immunoglobulins were ordered. Bone marrow biopsy demonstrated a hypercellular marrow (50% cellular) with a significant portion of the marrow cellularity being composed of blasts and promyelocytes. There were frequent bilobed cells and multiple Auer rods were noted in a subset of myeloid cells. The patient was diagnosed with Acute Myeloid leukemia (AML) with features worrisome for acute promyelocytic leukemia and further hematology oncology work up was suggested.
FISH was negative for the PML/RARA and RARA gene rearrangements. PML/RARA Intron 3 and Intron 6 breakpoint transcripts were not detected by quantitative real-time PCR. Cytogenetic studies showed normal female karyotype. No clonal numerical or structural abnormalities were observed. Studies for FLT3-ITD and FLT3 D835 mutations were negative. FISH studies for the 7q, 5q, 20q deletions, trisomy 8, RUNX1T1/RUNX1 gene fusion, KMT2A and CBFB gene break apart rearrangements were negative. Further testing included microarray analysis that showed a gain in copy number in the 11q23 region, spanning approximately 14.8 kb (Figure 1). This region contains exons 2-7 of the KMT2A gene. Next generation sequencing identified DNMT3A p.F640* and IDH1 p.R132C mutations.
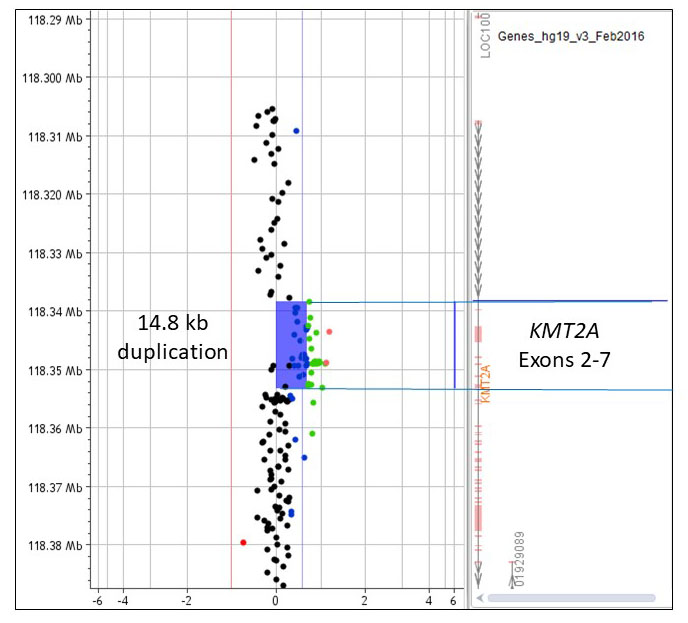
Figure 1. Array comparative genomic hybridization plot showing a gain in the 11q23.3 region corresponding to exons 2-7 of the KMT2A gene.
Based on the bone marrow and microarray findings, the patient was started on 7+3 induction chemotherapy with further plans for stem cell transplant. On follow-up day 14, bone marrow biopsy showed 10-20% cellularity with 4% blasts. Her hospital course was complicated by erythematous asymptomatic papular eruption which on biopsy was consistent with inflamed seborrheic keratosis possibly related to chemotherapy. Periodic acid-Schiff, Gomori methenamine silver, Gram, Fite and Ziehl Neelsen stains were negative for microorganisms. The patient further completed 2 cycles of consolidation chemotherapy and was found to have 2.5% blasts on bone marrow biopsy when she was scheduled for matched unrelated donor stem cell transplantation.