FINAL DIAGNOSIS
X-linked Alport Syndrome
DISCUSSION
Alport syndrome is a group of genetic diseases due to mutations in one of the alpha chains of Type IV collagen and may have an X-linked (85% of cases), autosomal recessive (10-15%) or autosomal dominant pattern of inheritance [1,2]. Type IV collagen is a component of normal glomerular basement membranes and structurally consists of a triple helix made up of three alpha chain monomers forming a heterotrimer. There are six different type IV collagen alpha chain isoforms (α1-α6), each encoded by a separate gene (COL4A1-COL4A6). COL4A1-COL4A4 are located on autosomal chromosomes with COL4A1 and COL4A2 on chromosome 13 and COL4A3 and COL4A4 on chromosome 2 while COL4A5 and COL4A6 are located on the X chromosome. The mature glomerular basement membrane is composed of the α3- α4- α5 heterotrimer of type IV collagen and mutations in these genes result in the clinical manifestations of Alport syndrome. X-linked Alport's syndrome is due to mutations in COL4A5 while the autosomal recessive variant is due to mutations in COL4A3 or COL4A4 [3].
Clinically, X-linked Alport syndrome usually presents in early childhood with hematuria in affected males [4]. Over time the patients will develop proteinuria and ultimately end stage renal disease. Depending on whether patients progress to end stage renal disease before or after the age of 30 years, X-linked Alport syndrome can be categorized into a juvenile or adult form. The juvenile form has been found to be associated with diffuse leiomyomatosis in 2-5% of families with this variant [5]. Other more common extrarenal manifestations of X-linked Alport syndrome include sensorineural deafness and ocular abnormalities. In female carriers of the syndrome, the presentation is much later and these patients have a more variable clinical course due to random inactivation of the X-chromosome or lyonization in females [6].
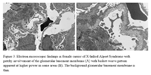
Histologically, if taken early in the disease progression, renal biopsies may demonstrate normal glomeruli with only occasional tubular red blood casts correlating with the clinical presentation of microscopic hematuria, and thin glomerular basement membrane present ultrastructurally. Over time, nonspecific thickening and wrinkling of the glomerular basement membrane will develop with occasional basement membrane splitting visible on Jones silver stains followed by segmental tuft sclerosis corresponding with the clinical development of proteinuria. Renal tubules will also begin to show changes consistent with progressive disease with vacuolization as a result of resorbed lipoprotein. In the interstitium, foam cells may be present at the corticomedullary junction arranged in linear rows within the corte [7]. Eventually, the disease evolves into chronic renal disease with chronic interstitial inflammation, tubular atrophy and interstitial fibrosis as was seen in our case. On routine immunofluorescence, as was demonstrated in our case, there may be nonspecific mesangial staining for IgG, IgM and C3 and as the patients develop focal segmental glomerulosclerosis focal segmental glomerular staining for IgM, C3 or C1q may be seen in these lesions7. Immunofluorescence evaluation using antibodies against the six different alpha chain isoforms of type IV collagen performed on frozen tissue from a renal biopsy from a patient with X-linked Alport syndrome will demonstrate loss of staining for α3, α4, and α5 chains in the glomerular basement membrane. In the presence of an α5 chain mutation, the assembly of type IV collagen is impaired so none of the three alpha chains are incorporated into the basement membrane [5]. Female carriers will have mosaicism with patchy loss of the three α chains alternating with areas of immunophenotypically normal glomerular basement membrane [8]. Ultrastructurally, early disease will only show a thinned basement membrane while later more advanced disease demonstrates the characteristic basket-weave appearance. Female carriers may have only patchy presence of the basket weave pattern (Figures 3A, 3B).
The differential diagnosis for Alport Syndrome includes thin glomerular basement membrane nephropathy. This disorder is also due to mutations in the α chains for type IV collagen, specifically the COL4A3 and COL4A4 genes, and is inherited in an autosomal dominant fashion. These disorders can be distinguished by their differing immunofluorescent patterns and characteristic family histories. Thin glomerular basement membrane nephropathy will have normal staining for type IV collagen α chains while X-linked Alport syndrome will have loss. Recent classifications have included thin basement membrane nephropathy under the category of autosomal dominant Alport Syndrome [3], emphasizing that these entities exist in the same family of disorders.
REFERENCES
![]() Contributed by Lara Berklite, MD and Sheldon Bastacky, MD
Contributed by Lara Berklite, MD and Sheldon Bastacky, MD