FINAL DIAGNOSIS
Transfusion Related Acute Lung Injury (TRALI).
DISUCSSION
Definition:
TRALI is a new lung injury, occurring within 6 hours of transfusion, unrelated to any competing etiology for acute lung injury and in the absence of circulatory overload.
Clinical Presentation:
TRALI typically presents as acute onset respiratory distress with hypoxemia, dyspnea, and tachypnea with concurrent tachycardia, fever and/or hypotension.
Pathogenesis:
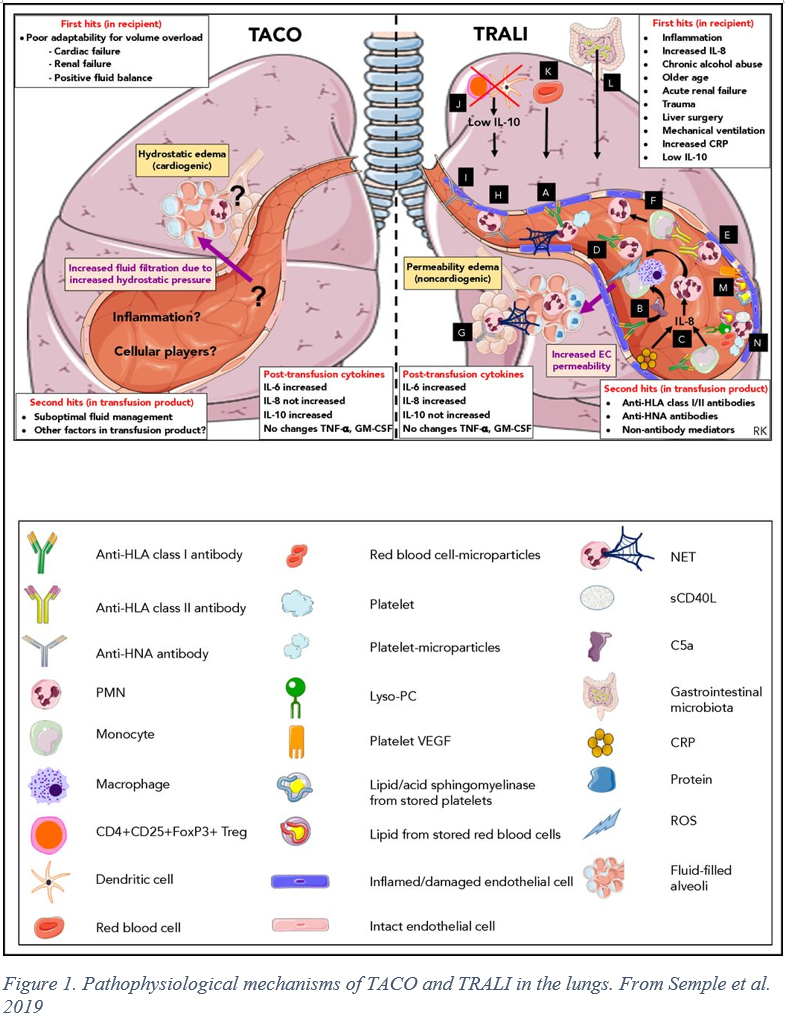
While the pathogenesis of TRALI is not completely understood, a 2-hit model is believed to underlie the disorder. The first hit consists of pre-existing clinical risk factors. These risk factors are reflective of an overall inflammatory state in the patient, with resultant priming of neutrophils, monocytes and endothelial cells that increase the risk of ARDS following transfusion. (1) The second hit is delivered with the infusion of the blood product. In immunologic TRALI, the second hit is caused by human neutrophil antigen (HNA) antibodies and/or HLA Class I/II antibodies. (2) Accordingly, transfusion from female donors, multiparity of the donor, or the donor having received past transfusions are risk factors for immunologic TRALI. The volume of transfused antibodies and strength of binding (eg MFI > 1500) were found to be increase the risk of TRALI in multivariate analysis controlling for other patient risk factors. (1) Some cases of TRALI are non-immunologic in origin, instead involving direct biological response modifiers targeting the vascular endothelium. (3) Ultimately, with either type, the permeability of the pulmonary microvasculature is altered, resulting in an exudate filling the interstitium and alveolar airspaces. (See Figure 1)

Case Immunologic Workup:
HLA typing was performed on the recipient and the donor was screened for the presence of anti-HLA antibodies.
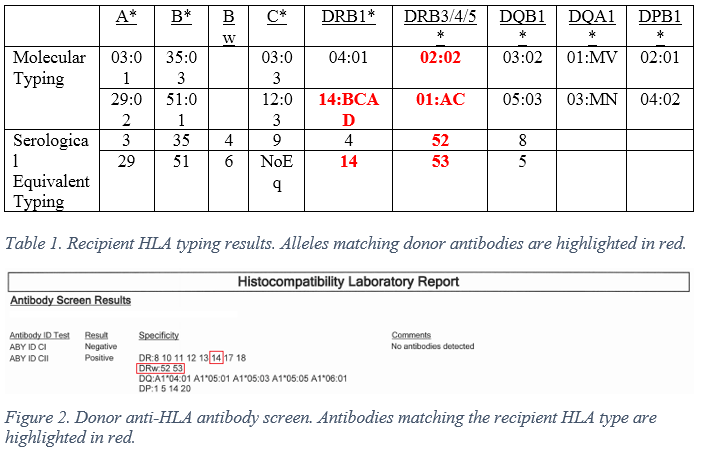
Initial results revealed three potential reacting antibodies in the donor, DR14, DR52 and DR53. The lab was contacted to determine the molecular typing used for detection of DR52 and DR53 antibodies and they were found to be DRB3*02 and DRB4*01 respectively, matching the allele groups present in the patient.

Solid phase HLA assay testing was performed to examine the strength of the antibodies in question, revealing two of the three candidates, DR14 and DR52, to be strongly reacting. Overall, the results of the immunologic testing are consistent with an immunologic etiology for the patients TRALI. Further investigation revealed that the donor had several risk factors for anti-HLA antibodies, being a multiparous woman and past blood transfusion recipient, but no history of causing transfusion reactions as a donor.
Diagnostic criteria:
Standardized definitions of TRALI were first codified in 2004 at the Canadian Consensus Conference and 2005 with the National Heart, Lung and Blood Institute Working group. (4, 5) These groups provided standard definitions of TRALI to facilitate research and a decision making frameworks while introducing the concept of possible TRALI (pTRALI), that is TRALI in patients with a concurrent ARDS risk factor. Earlier this year, a collection of international experts, the TRALI Delphi panel, convened to propose a redefinition of TRALI based on results from intervening years of research and incorporating redefinitions of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) that occurred during the Berlin consensus conference in 2012. (6) The resulting recommendation of the TRALI Delphi group is to classify cases as TRALI type I, with no ARDS risk factors, or TRALI type II, patients with ARDS risk factors or mild ARDS with deteriorating respiratory status due to transfusion. The consensus definition can be seen in the table below:
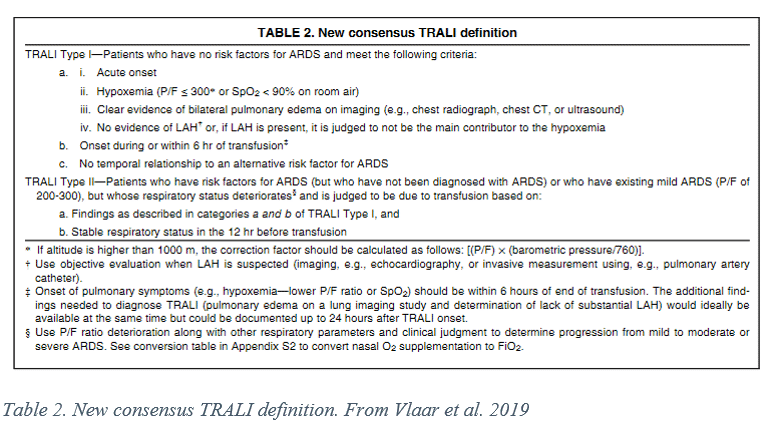
Of note is the fact that TRALI is a clinical diagnosis with no available biomarkers for confirmation. Cognate WBC antibody workup is of no clinical utility at the bedside due to the length of time it takes to receive results. Immunologic workup of donors involved is advised, however, for prevention of future reactions.
Treatment:
No specific therapy is available for TRALI. At the onset of symptoms, blood transfusion should be stopped and supportive care, including oxygen, intubation and fluid/pressor management, given to the patient.
Prevention:
Mitigation strategies such as testing multiparous females for donations of high-volume plasma products significantly reduced the risk of TRALI. Also, donors that are implicated in cases of TRALI are permanently deferred.
REFERENCES
![]() Contributed by Nathan Cook MD and Alesia Kaplan MD
Contributed by Nathan Cook MD and Alesia Kaplan MD