FINAL DIAGNOSIS
Plasmodium ovale.
DISCUSSION
Case:
An infectious disease consult provided further infectious and exposure history. The patient was originally from Southeast Asia and had three prior diagnoses of malaria, the most recent being 2008. The patient was unable to recall the parasite species or specific treatment regimen for the prior malaria diagnoses. In discussions with the CDC, the clinical team hypothesized that hepatic hypnozoites (see life cycle below) were reactivated by the recent trauma.
Malaria (1-3):
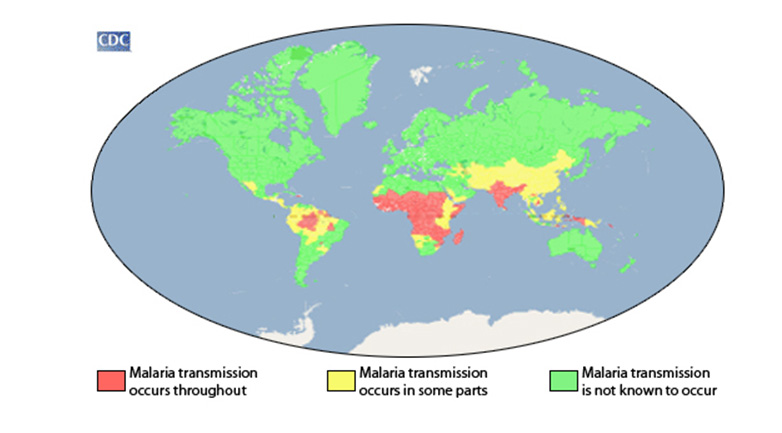
By global disease burden, Malaria represents the most important parasitic disease being transmitted in more than 100 countries inhabited by approximately 3 billion people. In 2015, according to the World Health Organization, there were 214 million new cases of malaria and an estimated 438,000 deaths worldwide. Five Plasmodium species (spp.) have been described to cause human malaria: Plasmodium falciparum, Plasmodium malariae, Plasmodium vivax, Plasmodium ovale and Plasmodium knowlesi. Malaria transmission occurs in tropical and subtropical regions of the world where the Anopheles mosquito can propagate, and Plasmodium spp. can complete its growth cycle within the mosquito vector (Figure 1).

Figure 1 Geographic distribution of malaria. Map displaying approximation of the parts of the world where malaria transmission occurs.
(From https://www.cdc.gov/malaria/about/distribution.html)
Vector and lifecycle (3, 4):
There are approximately 450 species of Anopheles mosquitoes currently categorized with a hundred of those capable of transmitting human malaria. However, only 30-40 of those species commonly transmit human malaria including Anopheles gambiae, one of the better described vectors.
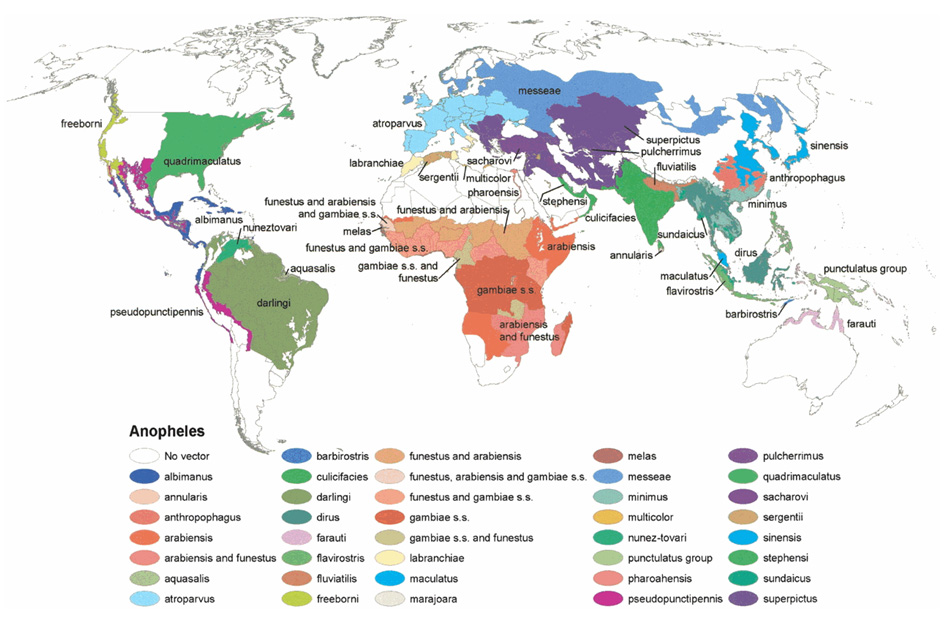
Figure 2 Global distribution of Anopheles species functioning as malaria vectors. Robinson projection displaying the dominant or potentially important malaria vectors.
(From http://www.ajtmh.org/docserver/fulltext/14761645/70/5/486f1_C4TT.gif)
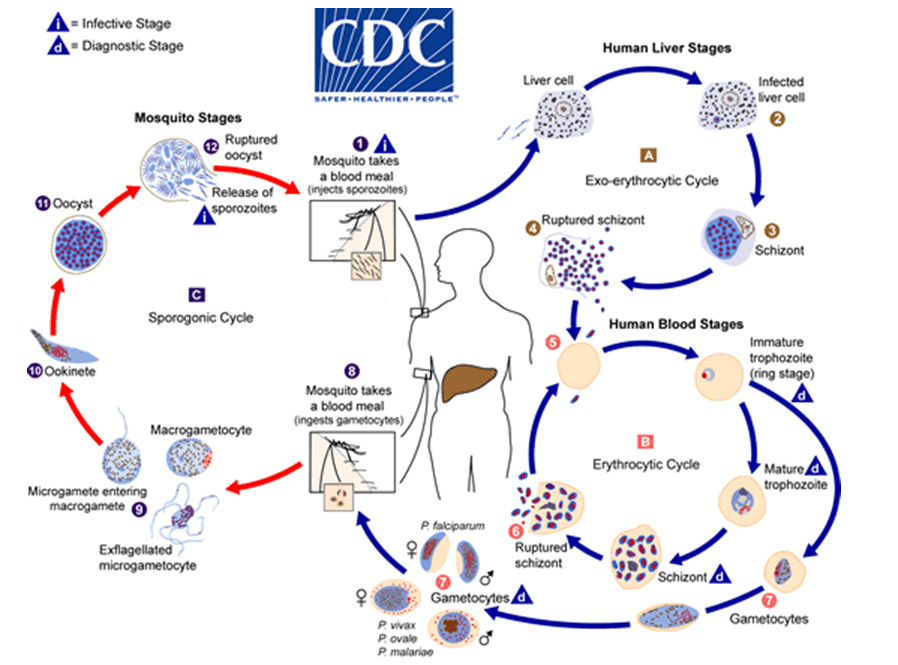
Figure 3 - Malaria parasite life cycle
https://www.cdc.gov/dpdx/malaria/modules/malaria_LifeCycle.gif
The malaria parasite life cycle involves two hosts.
Human:
A. Exo-erythrocytic schizogony
1. During a blood meal, a malaria-infected female Anopheles mosquito inoculates sporozoites into the human host. 2. Sporozoites infect liver cells. 3. Sporozoites mature into schizonts 4. Schizonts rupture and release merozoites.* In P. vivax and P. ovale, a dormant stage [hypnozoites] can persist in the liver and cause relapses by invading the bloodstream weeks, or even years later.
B. Erythrocytic schizogony
5. Merozoites infect red blood cells. 6. The ring stage trophozoites mature into schizonts, which rupture releasing merozoites. 7. Some parasites differentiate into sexual erythrocytic stages (gametocytes). (Blood stage parasites are responsible for the clinical manifestations of the disease.)
Anopheles:
C. Sporogonic cycle
8. The gametocytes, male (microgametocytes) and female (macrogametocytes), are ingested by an Anopheles mosquito during a blood meal. 9. While in the mosquito's stomach, the microgametes penetrate the macrogametes generating zygotes. 10. The zygotes in turn become motile and elongated (ookinetes) 11. The zygotes invade the midgut wall of the mosquito where they develop into oocysts. 12. The oocysts grow, rupture, and release sporozoites. 1. The oocytes make their way to the mosquito's salivary glands. Inoculation of the sporozoites into a new human host perpetuates the malaria life cycle. (Adapted from https://www.cdc.gov/dpdx/malaria/index.html)
Transmission (3, 5-7):
In addition to female Anopheles spp. transmitting Plasmodium spp. during a blood meal, vertical transmission has also been documented. In highly endemic areas, it is estimated that up to a third of infants are exposed to malaria in utero. Placental malarial infection is associated with low birth weight which is a significant factor in perinatal mortality.
Transfusion-transmitted infections of Plasmodium spp. occur in endemic areas with an incidence of up to 50 infections for every million blood transfusions. Though no approved test to screen blood products exist in the United States, only one case on average of transfusion-transmitted malaria transpires in the United States every two years (or an estimated 10 million blood transfusions). The FDA recommends deferring immigrants or travelers from areas with endemic malaria or any person with a diagnosis of malaria from donating blood for three years after exposure or symptoms resolve. However, due to asymptomatic persistence of Plasmodium spp., transfusion -transmitted malaria has been reported up to 44 years after last known exposure.
Clinical Presentation (3):
Infection with Plasmodium spp. may result in wide spectrum disease of ranging from asymptomatic carrier state to death. Following a blood meal with an infected Anopheles spp. mosquito, there is a 7-30 day incubation period prior to symptoms developing. Uncomplicated malaria presents with fever, chills, body aches and general malaise. Complicated (severe) malaria can lead to anemia, metabolic acidosis and end organ failure.
Infections with Plasmodium vivax and Plasmodium ovale can have relapses months to years after initial infection due to reactivation of dormant stage liver parasites called hypnozoites. Reactivation is most commonly associated with febrile illness.
Diagnosis (3):
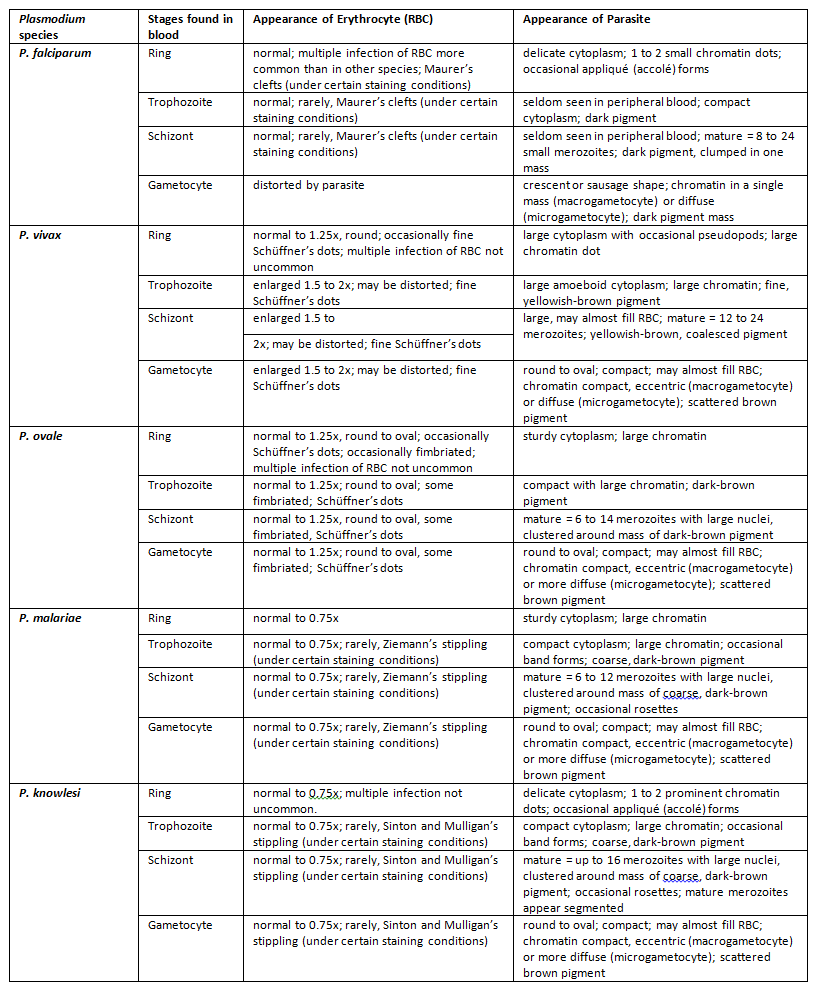
Identification of Plasmodium spp. infection is primarily performed through microscopy. For this case, microscopic review displayed round to oval infected erythrocytes with Schüffner dots without identification of crescent shape gametocytes (note: Schüffner dots are best appreciated with Giemsa stain). This prompted further evaluation of the Schizont form and quantitation of merozoites in order to differentiate Plasmodium vivax and Plasmodium ovale. Further analysis showed Schizonts with a maximum number of 12 merozoites favoring a diagnosis Plasmodium ovale in addition to oval distortion of erythrocytes. Refer to Figure 4 and Table 1 (at the end of the conclusion section) for further information regarding microscopic features used to distinguish Plasmodium species.
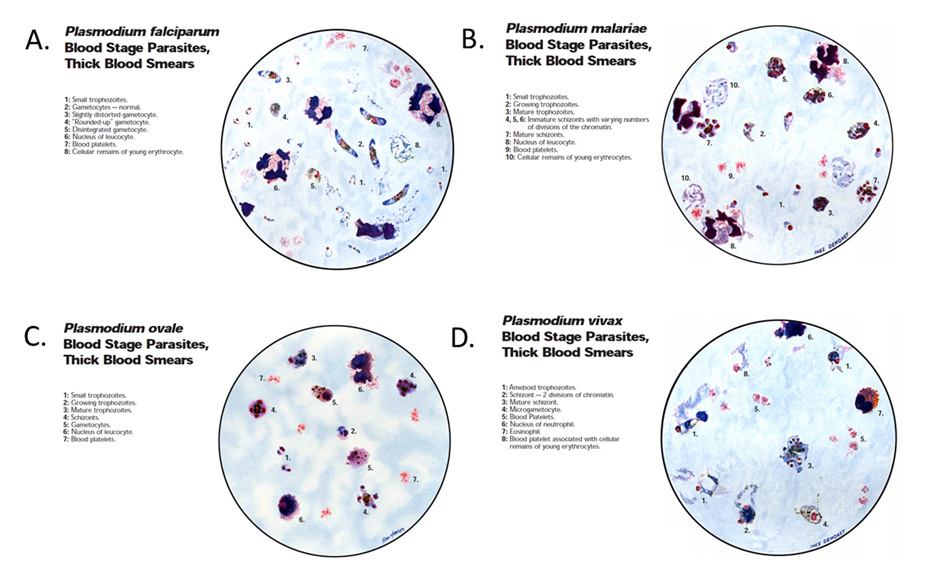
Figure 4 Microscopic features of Plasmodium spp.
Illustrations of microscopic features commonly found on thick blood smears in patients infected with (A) Plasmodium falciparum, (B) Plasmodium malariae, (C) Plasmodium ovale and (D) Plasmodium vivax. Illustrations from: Wilcox, A. Manual for microscopic diagnosis of malaria in Man. US Dept of Health, Education and Welfare. 1960.
Multiple rapid diagnostic tests have been developed and are lateral flow immuno-chromatographic antigen-detection tests. These tests are ideal in places where microscopy is not available; however, their costs have prohibited them from widespread use in malaria-endemic areas. In 2007, the US FDA approved the first rapid diagnosis test for hospital and commercial laboratories with recommendation that all rapid diagnosis tests be followed by microscopy.
PCR can be utilized for confirming Plasmodium species after infection is established by microscopy or rapid diagnostic test.
Serology tests identifying antibodies against malaria parasites can be performed utilizing either indirect immunofluorescent or ELISA to detect past exposure.
Treatment (3):
Based on disease burden (uncomplicated vs complicated), geographic area in which exposure occurred and age of patient, different treatment regimens and duration are recommended (https://www.cdc.gov/malaria/resources/pdf/treatmenttable.pdf). In addition to these regimens, infections with Plasmodium vivax and Plasmodium ovale should be treated with an additional 14 days of primaquine phosphate after initial treatment regimen.
Comparison with Babesia (8):
The patient's recent camping history raises clinical suspicion of babesiosis. Given the patient's recent splenectomy, it is important to rule out babesiosis as babesiosis can be life-threatening disease for asplenic patients. Babesia spp. infect red blood cells and can display ring forms, similar to Plasmodium spp. infections. However, Babesia spp. can be differentiated from Plasmodium spp. by lack of schizont, trophozoite, and gametocytes in human infection and lack pigmentation. Additionally, Babesia spp. can present in the form of tetrads (Maltese cross) and be observed outside of erythrocytes. For additional information regarding babesiosis, refer to CP webcase 982 (https://path.upmc.edu/cases/case982.html).
Conclusions:
Table 1 Microscopic features of Plasmodium species (from https://www.cdc.gov/dpdx/malaria/index.html)
REFERENCES
![]() Contributed by Tanner J. Freeman MD, PhD and Stephanie L. Mitchell, PhD, D (ABMM)
Contributed by Tanner J. Freeman MD, PhD and Stephanie L. Mitchell, PhD, D (ABMM)