
![]() Contributed by Li Liu, MD, PhD
Contributed by Li Liu, MD, PhD
CLINICAL HISTORY
The patient was a woman in her 80's with a history of solitary plasmacytoma and elevated serum kappa light chain. The solitary plasmacytoma occurred at the cervical spine 10 years ago and then at the right supraclavicular area 3 years ago, and were treated by radiation therapy both times. Her bone marrow biopsy showed a small cluster of kappa-restricted plasma cells consisting of less than 5% of total cellularity after her second radiation therapy. She has been followed up routinely by PET/CT scan, serum protein electrophoresis (SPE) and immunofixation electrophoresis (IFE). SPE and IFE on serum samples have not detected monoclonal antibodies to date.
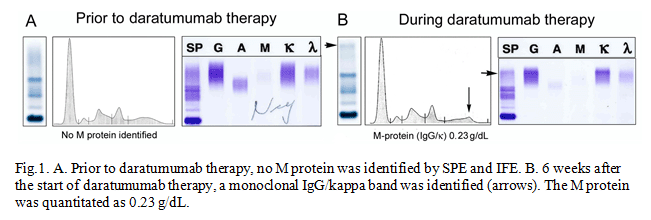
Her condition has been stable and was not on any chemotherapy until she experienced right thigh pain. A PET/CT scan showed an increase in focal uptake of 18F- fludeoxyglucose in the right distal femur. The distal femur bone lesion was not amenable to radiation therapy due to the close proximity to the knee joint. At the same time, her serum free kappa light chain level was increasing. These results indicated that her disease had been slowly progressing. Systemic treatment was considered to prevent further disease progression and complications from the bone lesions. Considering her age and side effects of different treatment plans, a newly FDA-approved drug for refractory multiple myeloma, daratumumab, was selected and administered. On her next routine follow-up, a monoclonal IgG/kappa band appeared on the SPE and IFE with an M-protein level of 0.23 g/dL. Her SPE, IFE and M-protein and serum kappa and lambda light chain results are shown below.
LABORATORY RESULTS
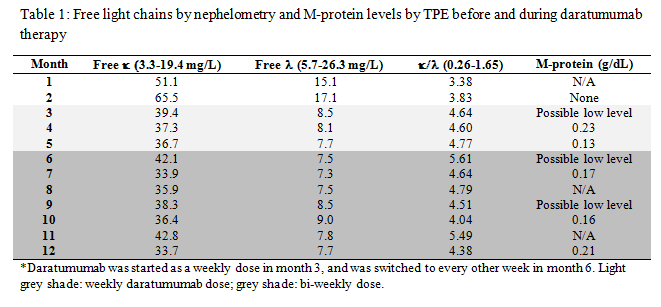
DIFFERENTIAL DIAGNOSIS
Plasmacytoma A plasmacytoma is a localized solitary mass of neoplastic plasma cells either in the bone or in various soft tissue sites without disseminated bone marrow involvement. Solitary plasmacytomas of the bone (SPB) accounts for 2-5% of all plasma cell neoplasms and occurs most commonly in the vertebrae, while extramedullary plasmacytomas (EMP) arise in the soft tissue, most commonly in the head and neck, and accounts for approximately 3% of all plasma cell neoplasms (1-3). Most SPB patients present with skeletal pain or a pathologic fracture of the affected bone. Diagnosis of SPB can be made when there is radiographic evidence of a solitary bone lesion and monoclonal plasma cell infiltrate on tissue biopsy. In addition, multiple myeloma is ruled out by the absence of bone marrow involvement and the clinical features of myeloma; absence or low concentration of serum or urine monoclonal protein; and normal levels of polyclonal immunoglobulins (4). The recommended treatment of SPB is local radiation therapy. Plasmacytoma often progresses to multiple myeloma (MM). The progression from SPB to MM ranges from 48% to 66% with the median time to progression of 2 years, but could be as long as 15 years. Older age at initial presentation and M protein positivity have a higher propensity for progression to MM (5). Conversion from EMP to MM is less frequent than that from SPB. It has been reported that 13% of multifocal EMPs progressed to MM (6). Because of the progression to MM, lifelong evaluation for disease progression is necessary with all plasmacytomas (7). This includes frequent bone marrow aspirations, skeletal surveys, and serum and urine protein electrophoresis to detect the presence of M proteins. Once MM is present, systemic treatment with chemotherapy is recommended.
Monoclonal gammopathy of undetermined significance (MGUS)
MGUS is the most common plasma cell disorder. It occurs in 3% of healthy individuals older than 50 years and over 5% of those older than 70. It is an asymptomatic clonal precursor lesion which does not always progress to overt malignancy. The diagnosis of MGUS can be made when serum M-protein is less than 30 g/L, bone marrow clonal plasma cells are less than 10%, and there are no lytic bone lesions or myeloma related organ or tissue damage.
MGUS has a small risk of progressing to MM or lymphoproliferative disorder, with the risk of progression ranging widely from 0.6 to 3.4 percent/year according to the initial value of serum monoclonal protein (8). Conversely, studies have suggested that virtually all patients diagnosed with MM had a preceding MGUS, with 75 percent having a detectible M-protein ?8 years prior to the diagnosis of MM (9-10). Therefore, MGUS patients should be monitored for disease progression and for potential complications. There is no known role for chemotherapy in the management of MGUS, although trials are ongoing to determine if the early use of drugs and other agents (eg, lenalidomide) can delay the progression of MGUS.
Multiple myeloma
MM is a multifocal, bone marrow-based plasma cell malignancy. It usually associates with monoclonal immunoglobulins in serum or urine, with IgG being the most common isotype, accounting for 60% to 70% of all MM cases. The diagnosis of myeloma is based on the presence of at least 10% clonal bone marrow plasma cells and monoclonal protein in serum or urine. In patients with true nonsecretory myeloma, the diagnosis is based on the presence of 30% monoclonal bone marrow plasma cells or a biopsy-proven plasmacytoma (11). Myeloma is classified as asymptomatic or symptomatic, depending on the absence or presence of myeloma-related organ or tissue dysfunction, including hyperCalcemia, Renal insufficiency, Anemia, Bone lesions: CRAB. Although MM is still an incurable disease, significant improvement in survival rate and quality of life has been achieved over the past two decades due to the introduction of newer agents, including immunomodulatory drugs and proteasome inhibitors. However, after periods of remission, the disease will eventually relapse and progress, with subsequent relapses occurring at increased frequency and less responsive to frontline therapies (12). The patient is considered to have a refractory disease when not responding to therapy any more. The treatment of refractory multiple myeloma is revolutionized by the introduction of therapeutic monoclonal antibodies (mAb) approved by the Food and Drug Administration (FDA) within the past two years, including daratumumab and elotuzumab.
Daratumumab
Daratumumab, a fully human IgG1/kappa mAb against plasma cell surface antigen CD38, induces tumor cell death through several different mechanisms. Early phase clinical trials have demonstrated the effectiveness of daratumumab against refractory/relapsed myeloma with an objective response rate (ORR) of 88% when using in combination with other agents, and 36% when using as a single agent (13).
As daratumumab and other therapeutic mAbs gain popularity in treating multiple myeloma, it also complicates the monitoring of myeloma patients. Multiple myeloma is typically monitored by the detection of the disease-associated M-protein by SPE and IFE. Complete or partial response requires the elimination or 50% reduction of detectable M-protein in peripheral blood, respectively. If the use of therapeutic monoclonal antibodies results in a detectable monoclonal protein by electrophoresis methods, it will interfere with the interpretation of these tests and may cause misdiagnosis and/or misclassification of clinical responses. Pharmacokinetic studies demonstrate that, following a weekly schedule at the recommended dose of 16 mg/kg, the mean peak plasma concentration of daratumumab is approximately 915 g/mL (0.09 g/dL) at the end of the dosing interval (14), far above the detection limit of the SPE and IFE (100-400 g/mL). In vitro spiking studies have shown that daratumumab can be readily detected at concentrations above 0.05 g/dL by IFE, and migrates toward the end of the zone (15), confirming its ability to complicate the interpretation of SPE and IFE.