FINAL DIAGNOSIS
The patient consumed a bath salt and this class of drugs is detected by a urine comprehensive drug screen (UCDS) using a Gas Chromatography-Mass Spectrometer (GC-MS).
Designer Drugs
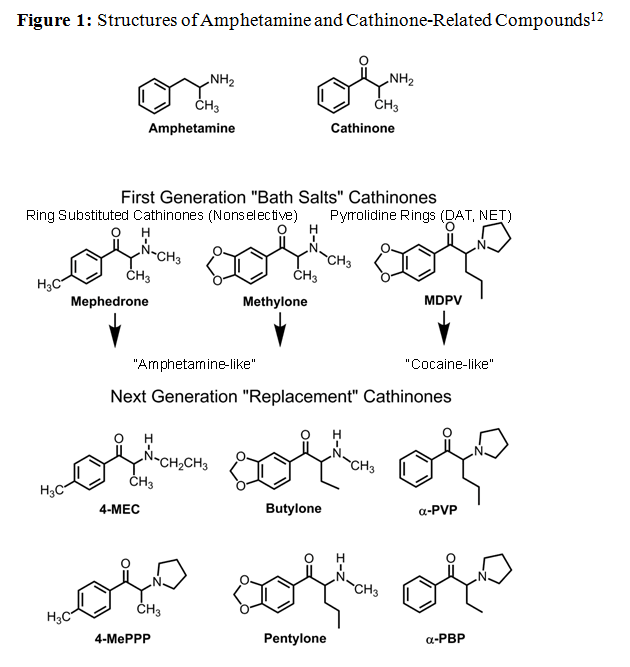
The current major classes of designer drugs are bath salts (synthetic cathinones) and K2 or Spice (synthetic cannabinoids), although hallucinogens and sedatives also exist1. They are globally manufactured on an industrial scale and their synthesis involves chemically modifying the pharmacophores of parent compounds with known psychoactive properties2. Although the parent molecules are illegal in most countries, these drugs are technically novel entities and therefore may not be subject to the laws regulating the sale and use of controlled substances3. Also, by being labeled as "not for human consumption," they circumvent health and safety regulations1,4.
Bath Salts
Khat (Catha Edulis) is a plant from the horn of Africa whose leaves are chewed or brewed in tea to provide mild stimulant and euphoric effects6. Its predominant active compound, cathinone, is a beta-keto amphetamine that was first isolated and identified in the 1960's5. Although numerous cathinone-related pharmaceutical products have entered the market, all except for bupropion have been removed due to toxicity3. Synthetic cathinone abuse began to steadily rise in 2003, and by 2011 there were 22,904 ER visits related to them in the US, prompting the DEA to list bath salts as a "chemical of concern"6. Subsequent legislation, such as 2012's Synthetic Drug Abuse Prevention Act, widened the net of 1986's Federal Analogue Act by listing 26 types of synthetic cathinones and cannabinoids as Schedule 13.
The mechanisms of action for their psychoactive effects can roughly divided into two groups (see Figure 1). Ring-substituted cathinones, such as methcathinone, mephedrone, and methylone, nonselectively interact with monamine transporters in a manner similar to that of amphetamines1. They reverse the normal flow of material through the transporter and cause neurotransmitter efflux into the synapse. Pyrrolidinophenones such as Methylenedioxypyrovalerone (MDPV) selectively block norepinephrine and dopamine transporters in a manner similar to cocaine2,7. They prevent neurotransmitters uptake from the synapse. Studies are currently being performed to determine the pharmacodynamics and pharmacokinetics for most prevalent compounds7,8,9.

Presentation
Many patients with acute bath salt intoxication have overstimulation of the sympathetic nervous system and present in a state of excited delirium, characterized by altered mental status with extreme agitation, aggression, violent behaviors, anxiety, delusions, or seizures4,10. They can also exhibit systemic findings of tachycardia, hyperthermia, diaphoresis, mydriasis, and hypertension, which are consistent with sympathomimetic syndrome toxidrome. Some patients, including this one, also present with increased WBC's. Several studies have shown that this may be due to physiological stress from increased peripheral catecholamines and cytokines11.
Rare cases of myocardial infarction and death have been reported4; however one of the most common secondary complications is rhabdomyolysis3,4. The mechanism may be related to excessive exertion due to tremors, myoclonus, or psychomotor agitation, as well as the sympathetic system's impairment of evaporative cooling10,12,13. The muscle necrosis leads to the release of cellular products such as creatine phosphokinase (CPK), myoglobin, lactate dehydrogenase, and aminotransferases (AST and ALT), and nucleosides (precursors to uric acid)14,15. Initial CPK levels under 15,000-20,000 are associated with a decreased risk for acute tubular necrosis15. The myoglobin released into the serum is filtered by the glomerulus and broken down into constituents that constrict renal vasculature, as well as obstruct and damage renal tubules14,16. These excreted products are detected by urinalysis. Heme pigment provides a positive dipstick test for blood with a concurrent low microscopic RBC count, globin proteins are recognized as proteinuria, and damaged tubules are recognized as hyaline casts15.
Diagnosis
Clinicians must quickly narrow their differential diagnosis when trying to stabilize patients with acute intoxication. Unfortunately, histories obtained from altered, seizing, or unconscious patients are unreliable or unobtainable. Clinicians will, therefore, take a sample of urine to detect the presence of drugs and metabolites.
If one of the routine drugs of abuse is suspected, clinicians will order a urine drug screen (UDS), which utilizes competitive homogenous EIA's performed by the enzyme-multiplied immunoassay technique (EMIT) on the VIVAE (Siemens). There is a different EIA kit for each drug class tested: amphetamines, benzodiazepines, opiates, barbiturates, cocaine metabolites, phencyclidine, THC metabolites, methadone, and propoxyphene. EIA's are ideally suited for initial screening because they are automated and generally provide results in 5-10 minutes. Unfortunately, there are currently no FDA-approved EIA kits for bath salts. If there is a high clinical suspicion for bath salts, clinicians will order a UCDS which includes the initial EIA screen and a subsequent sample analysis by a qualitative GC-MS test (see Figure 2).
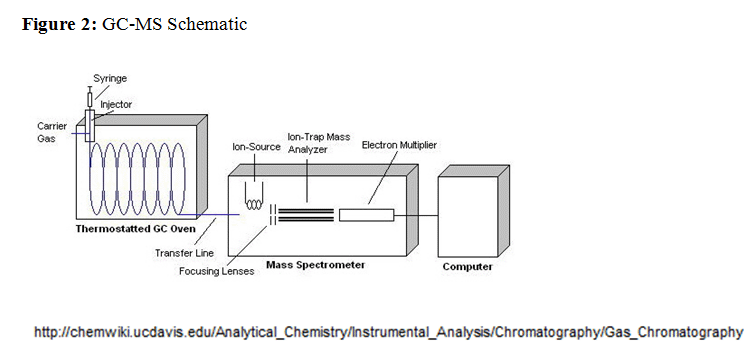
The GC-MS test is labor intensive and takes around two hours to perform. The first step involves extracting the sample analytes from urine using charcoal and methylene chlorine. The extract is then dissolved in methanol and injected into the GC-MS. The GC column consists of a stationary phase, and a gaseous mobile phase through which the sample moves. The rates at which the sample's constituents travel is primarily determined by their relative affinity for stationary phase particles. At the end of the column the individual components are detected and then identified based on their retention times (see Figure 3). Upon exiting the GC column, analytes enter the MS sample inlet and proceed toward a hard ionization source that bombards them with electrons in order to form a reproducible set of ionized fragments. These ions are separated based on their mass-to-charge ratio by a quadrupole mass analyzer, before passing through a detector. The sample's spectrogram (see Figure 4) is then matched to molecules in the instrument's library (see Figure 5).
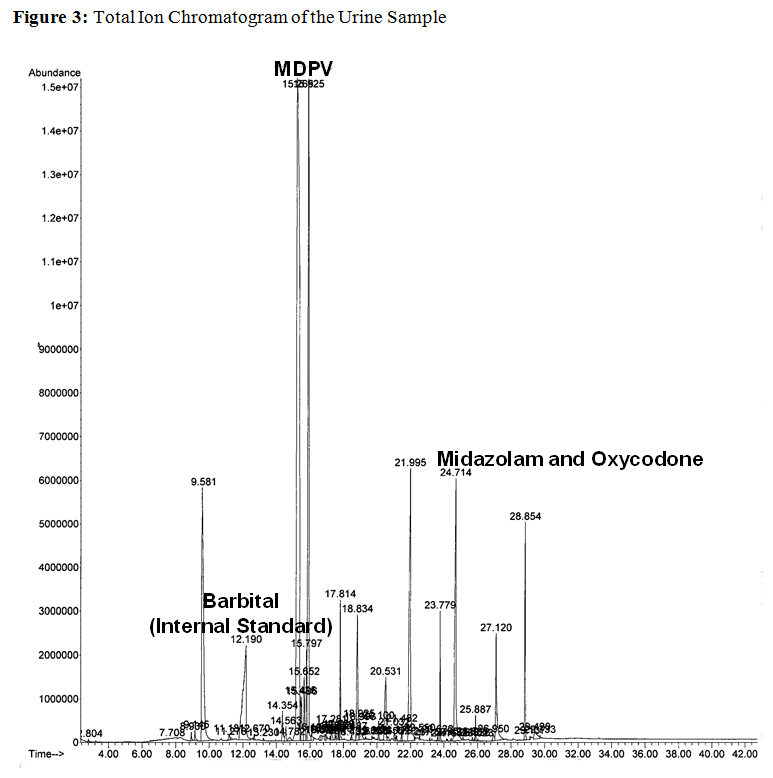
In the total ion chromatogram (TIC), the x-axis values represent the retention times for each of the analytes, and the y-axis values represent the relative abundance of the analytes compared to the internal standard (barbital). The peak at 15.9 corresponds with the bath salt MDPV, and this finding is consistent with the patient's acute presentation. At 24.1, there are distinct peaks corresponding to midazolam and oxycodone, which appear to overlap due to low resolution of TIC (Figure 3). The detection of a benzodiazepine, such as midazolam, was expected due to the patient's treatment and positive UDS. In contrast, the concurrent detection of oxycodone is indicative of polysubstance abuse, and shows the lower relative sensitivity of regular opiate EIA tests for semi-synthetic opiates.
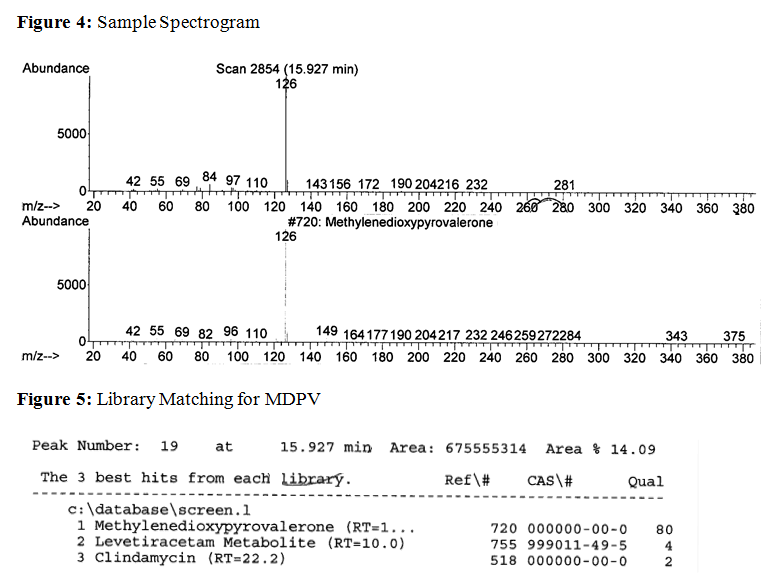
On the spectrogram, the x-axis values represent the mass-to-charge ratios for an analyte's ionized fragments, and the y-axis values represent the relative abundance of these fragments. The top spectrogram corresponds to the analyte from the patient's sample and the bottom spectrogram corresponds to the library reference for MDPV. The computer's library indicates that the analyte matches best with MDPV.
Treatment
Clinical management of acute intoxication from bath salts is predominantly supportive care and there are no validated guidelines or specific antidotes10. Agitation and excessive sympathetic stimulation are initially treated with benzodiazepines because they reduce heart rate, blood pressure, neural stimulation, muscular activity, and prevent seizures13. If the patient's agitation continues to prevent their treatment, poses a danger to staff4, or leads to the loss of protective airway reflexes, sedation and intubation are generally recommended10. Antipsychotics must be used with caution because they may interfere with heat dissipation and lower seizure thresholds12. Additional measures include IVF's, aggressive cooling for hyperthermia, a low stimulation environment, and antihypertensives4,10. IVF's are given to minimize prerenal ischemic injury due to the third spacing of extracellular fluid into muscle, or decreased intake by the patient prior to presentation15. They maintain renal perfusion and decrease the formation of tubular casts secondary to rhabdomyolysis17. For refractory hypertension, nitroprusside or phentolamine is administered, while beta-blockers are avoided due to the fear of unopposed alpha stimulation12.
Patient Follow-up
The patient presented with the behavioral symptoms of excited delirium and the results of his physical exam and vitals were consistent with the sympathomimetic toxidrome. His initial laboratory values showed elevated serum CPK, lactate, and transaminase concentrations consistent with rhabdomyolysis. He also had a slightly elevated serum BUN/Cr ratio, urine hyaline casts, and myoglobinuria which are consistent with mild acute kidney injury due to acute tubular necrosis.
His UCDS was positive for MDPV, midazolam, and oxycodone, which indicate bath salt intoxication, subsequent benzodiazepine treatment, and additional opiate abuse. Through supportive management, his symptoms subsided, with an overall improvement of his lab results. He was successfully extubated on day six of treatment, and discharged the following day with a CPK <500.
REFERENCES