FINAL DIAGNOSIS
Sclerosing hemangioma
DISCUSSION
Sclerosing hemangioma, first described in 1956 by Liebow and Hubbell,(1) is predominantly found in middle-aged women. Patients are often asymptomatic with the lesion discovered incidentally. The incidence is higher in East Asian patients and is rare in Western population. Imaging studies show a well-circumscribed mass with marked contrast enhancement on CT.
The tumors are usually solitary, peripheral, and encased by the lung parenchyma, although they can rarely be found in the pleura and mediastinum (2). Metastases to the regional lymph nodes have been reported in a small number of cases (3,4) and this does not adversely affect prognosis (4).
On gross inspection, the mass is grey to tan-yellow with areas of hemorrhage. Microscopically, sclerosing hemangiomas have a characteristic appearance and contain two types of cells, round stromal and surface cuboidal. There are four architectural patterns: papillary, sclerotic, solid, and hemorrhagic (2). Papillary areas have fibrovascular cores with stromal cells and lined by cuboidal cells. Sclerotic areas have dense hyaline collagen, and solid areas show sheets of round cells with scattered small tubules. Hemorrhagic areas demonstrate large blood-filled spaces, cholesterol clefts, hemosiderin deposits, and foamy macrophages.
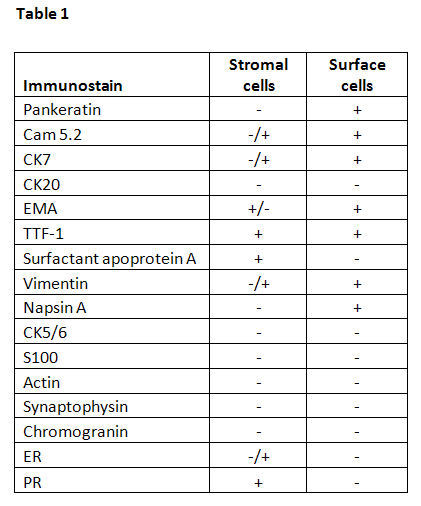
Stromal and surface cells have slightly different immunoprofiles as summarized in Table 1 (5,6,7,8). Both cell types are positive for EMA and TTF-1. Stromal cells may be positive for estrogen receptor (ER) and progesterone receptor (PR). Surface cells are positive for surfactant apoprotein A and pankeratin.

The cell of origin of this tumor was a subject of debate for many years. It was regarded as a vascular neoplasm based on morphologic appearance and hence called sclerosing hemangioma. Other postulated cells of origin included mesenchymal, mesothelial and neuroendocrine. Based on immunophenotypic data, the tumor is currently thought to be derived from primitive, undifferentiated respiratory epithelium (5,9). Based on these observations it was suggested to rename this neoplasm sclerosing pneumoyctoma. Molecular data are consistent with neoplastic nature of this lesion (9,10). Aberrant mTOR signaling may play in role in its development (11).
The differential diagnosis primarily includes papillary adenocarcinoma and carcinoid. Tumors with clear cell cytoplasm, both primary and metastatic such as renal cell carcinoma and clear cell carcinoma of the lung should also be considered in the differential diagnosis. Sclerosing hemangioma can generally be differentiated from other entities by its bland cytology, dual cell population and its characteristic immunoprofile.
REFERENCES
![]() Contributed by Alicia M. Hunt, MD, and Sanja Dacic, MD, PhD
Contributed by Alicia M. Hunt, MD, and Sanja Dacic, MD, PhD